Abstract
Human rhinovirus (RV) is the major cause of common cold, and it also plays a significant role in asthma and asthma exacerbation. The airway epithelium is the primary site of RV infection and production. In contrast, monocytic cells (e.g., monocytes and macrophages) are believed to be nonpermissive for RV replication. Instead, RV has been shown to modulate inflammatory gene expressions in these cells via a replication-independent mechanism. In the study presented here, replication of RV16 (a major-group RV) was found to be significantly enhanced in monocytes when it was cocultivated with airway epithelial cells. This effect appeared to be mediated by secretory components from epithelial cells, which stimulated RV16 replication and significantly elevated the expression of a number of proinflammatory cytokines. The lack of such an effect on RV1A, a minor-group RV that enters the cell by a different receptor, suggests that intercellular adhesion molecule 1 (ICAM1), the receptor for major-group RVs, may be involved. Indeed, conditioned media from epithelial cells significantly increased ICAM1 expression in monocytes. Consistently, ICAM1 overexpression and ICAM1 knockdown enhanced and blocked RV production, respectively, confirming the role of ICAM1 in this process. Thus, this is the first report demonstrating that airway epithelial cells direct significant RV16 replication in monocytic cells via an ICAM1-dependent mechanism. This finding will open a new avenue for the study of RV infection in airway disease and its exacerbation.
Keywords: airway, epithelium, rhinovirus, ICAM1
Clinical Relevance
We demonstrate, for the first time, that epithelial secretions direct robust RV replication in monocytes via significantly increased ICAM1. This new information will advance our understanding of the interaction between the airway epithelium and inflammatory cells in the context of RV infection and RV-induced disease exacerbation.
Human rhinovirus (RV) is a small (30 nm in diameter), nonenveloped, positive-stranded RNA virus with a genome of ∼7.2 Kb (1). The modern classification method (based on sequence similarity) divides RV into three genetically distinctive groups (A, B, and C) (2), whereas the traditional classification is based on the cellular receptors for viral entry (1). In this system, RV can be divided into two groups: the major group, which enters cells via an intercellular adhesion molecule 1 (ICAM1)-mediated process, and the minor group, which binds to the family of low-density lipoprotein receptors (LDLRs) for cellular entry. Notably, because of the functional conservation between humans and mice (i.e., LDLR), or the lack thereof (i.e., ICAM1), only minor-group RVs can infect mice or mouse cells.
RV infection is the major cause of common cold in healthy individuals (1). It also plays a significant role in the development (3, 4) and exacerbation (5–9) of asthma. The epithelium is the primary site of RV infection and replication in human airways (2). RV was shown to infect ex vivo epithelial cell cultures derived from human trachea (10), bronchus (11), submucosal gland (12), and lung alveoli type II pneumonocytes (13). In contrast, RV entered, but did not replicate, inside human monocytes or macrophages (14). RV was found to modulate the expression of various cytokines (e.g., IL-6 [15], TNF-α [15], IL-8 [16], CCL2/MCP1 [17, 18], CXCL10/IP10 [19, 20], IFNs [21], and CXCL11 (19)) in human monocytes or macrophages in the absence of viral replication.
Johnston and colleagues (16) were the first to report a low-grade productive replication of RV9, a major-group RV, in THP-1, a human monocytic cell line. Later, the same group demonstrated that RV16, another major-group RV, could replicate in macrophages that were derived from THP-1 using a phorbol myristate acetate (PMA) protocol (22). However, the molecular mechanism underlying this conversion from non- or low-permissive to permissive status remains unclear. In the present study, we found that soluble factor(s) from airway epithelial cells significantly increased ICAM1 expression on monocytic cells, and the elevated ICAM1 expression was responsible for robust RV16 replication in these cells. Because of the abundance of monocytic cells (e.g., monocytes and macrophages) in inflamed airways, our data may shed some light on the role of these cells in RV-induced diseases and disease exacerbations.
Materials and Methods
Viruses and Antibody
RV16 and RV1B stocks were amplified and purified based on a previously published protocol (23). Viral titers were determined by plaque assay as described previously (23). The anti-RV16 monoclonal antibody was a gift from Dr. Waiming Lee (University of Wisconsin, Madison, WI). To make replication-deficient RV16 (UV-RV16), stocks of RV16 were UV irradiated as described previously (11). Briefly, 1 ml of RV stock solution containing 5 × 108 RV16 was exposed to 200 μW cm−2 UV light for 10 min on ice.
Cell Culture, Conditioned Media, and RV Infection
THP-1 and A549 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultivated on regular tissue-culture dishes in RPMI media plus 10% fetal bovine serum. For coculture (CC), THP-1 and A549 cells were cultivated at a 1:1 ratio in the same culture dish and media. Human bronchial tissues were obtained from the National Disease Research Interchange according to an approved protocol. Primary human bronchial epithelial (HBE) cells were cultivated as described previously (11) in Ham’s F12/Dulbecco’s modified Eagle’s medium (1:1) supplemented with eight factors: insulin (5 mg/ml), transferrin (5 mg/ml), epidermal growth factor (10 ng/ml), dexamethasone (0.1 mM), cholera toxin (10 ng/ml), bovine hypothalamus extract (15 mg/ml), bovine serum albumin (0.5 mg/ml), and all-trans-retinoic acid (30 nM). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy individuals with consent at the University of Arizona Medical Center under an approved IRB protocol. To prepare conditioned media (CM) from epithelial cells, uninfected epithelial cells (A549 or primary HBE) were cultivated for 24 h in serum-free or growth-factor–free media, and the media were collected and filtered through a 0.2 μm sterile filter (Corning Inc., Corning, NY). The RV infection protocol was described previously (11). Briefly, RV was directly diluted into culture media at a multiplicity of infection (MOI) of 10 and incubated for the desired time. Then, the cells were washed and incubated in fresh media or CM at 35°C for 24 h. At the time of sample collection, the cells were washed three times in PBS to remove viral particles in the media. The lack of viruses was confirmed by PCR assay of the final wash (11). For CC, RV was added to the CC as described above and incubated for 24 h. At the time of harvest, epithelial cells and monocytes were separated and thoroughly washed before RNA or protein collection. To quantify infectious viral particles, a modified 50% tissue culture infective dose (TCID50) protocol was developed. In contrast to the common protocol (11), in which RV-infected cells are determined by a subjective judgment regarding morphological changes, we calculated TCID50 by immune staining infected cells using an anti-RV16 antibody.
RNA Extraction, cDNA Synthesis, and Real-Time Quantitative PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was prepared from 2 mg of total RNA and further diluted to 100 μl with water. Then, 2 μl of the diluted cDNA was analyzed using SYBR Green PCR Master Mix on a Veriti Thermal Cycler (Thermo Fisher, Grand Island, NY). Primers were used at 0.2 mM. The relative amount of mRNA in each sample was calculated based on the ΔΔCt method using the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Results were calculated as fold induction over control (24). For RV16 quantification, strand-specific RT-PCR was used to amplify either RV16 mRNA or RV16-negative strands as previously described (25). Quantitative PCR was then used for quantification. The authenticity of the PCR product was confirmed by DNA sequencing. The primers used are listed in Table 1.
Table 1.
PCR Primers
| Gene | Primer |
|---|---|
| GAPDH | Forward CAATGACCCCTTCATTGACC |
| Reverse GACAAGCTTCCCGTTCTCAG | |
| ICAM1 | Forward CCTTCCTCACCGTGTACTGG |
| Reverse AGCGTAGGGTAAGGTTCTTGC | |
| RV16 | Forward GCTGTGCAGTTGGATGTGAT |
| Reverse AAAGCCATGATGCAATCTCC | |
| RV1A | Forward TGGACCAAGAAACCTTCACAG |
| Reverse GTGCCACCTCAGGTGACATAA | |
| TNF-α | Forward TGATGCCACCAGATTTGACTG |
| Reverse GTGCGCTTGAATGTCAGGAAT | |
| IL-6 | Forward GTAGTGAGGAACAAGCCAGAGC |
| Reverse TCAGGGGTGGTTATTGCATCTA | |
| IL-8 | Forward AGCTCTGTGTGAAGGTGCAGT |
| Reverse TGGTCCACTCTCAATCACTCTC | |
| IFN-β | Forward ATTGCCTCAAGGACAGGATG |
| Reverse GCTGCAGCTGCTTAATCTCC | |
| CXCL10 | Forward TCCACGTGTTGAGATCATTGC |
| Reverse TCTTGATGGCCTTCGATTCTG | |
| CCL20 | Forward ATTTATTGTGGGCTTCACACG |
| Reverse CCAAGTCTGTTTTGGATTTGC | |
| CCL2 | Forward ACTCTCGCCTCCAGCATGAA |
| Reverse TTGATTGCATCTGGCTGAGC | |
| CXCL2 | Forward CGCCCAAACCGAAGTCAT |
| Reverse GATTTGCCATTTTTCAGCATCTTT |
Definition of abbreviations: CCL, chemokine (C–C motif) ligand; CXCL, chemokine (C–X–C motif) ligand; ICAM1, intercellular adhesion molecule 1; RV, human rhinovirus.
Western Blot
Total cellular protein was collected based on previously described methods (20). Anti-ICAM1 and anti-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Equal protein loading was confirmed by staining of the anti-actin antibody. Biological duplicates are shown in a representative image, and experiments were repeated at least twice.
Lentivirus Production and Transduction
pLX304-ICAM1 plasmid was purchased from the DNASU Plasmid Repository at Arizona State University (Phoenix, AZ). Packaging and titration of the recombinant lentiviral vector were performed as previously described (26). Briefly, 293T cells were transfected overnight with pLX304-ICAM1, VSVG, and Δ8.7 using Lipofectamine 2000 (Thermo Fisher). The cells were then treated with 10 μM sodium butyrate for 8 h and left to recover overnight. Supernatants were collected and pooled together. Viral particles were purified and concentrated using polyethylene glycol and stored at −80°C. Lentiviral particles containing randomized control small hairpin RNA (shRNA) and shRNA against ICAM1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monocytes were infected at an MOI of 10.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde and then either permeated with Triton X-100 or left untreated (nonpermeated). Intracellular RV16 protein was determined by immunofluorescence staining using anti-RV16 antibody. Alexa 488–conjugated secondary antibody (Thermo Fisher) was used to obtain fluorescence images by confocal microscopy (LSM 510 meta; Carl Zeiss, Thornwood, NY).
Statistical Analysis
Experimental groups were compared using a two-sided Student’s t test, with the significance level set as P < 0.05. When data were not distributed normally, significance was assessed with the Wilcoxon rank-sum test, and P < 0.05 was considered to be significant.
Results
Production of RV16 Was Significantly Enhanced in Monocytes when It Was Cocultivated with Airway Epithelial Cells
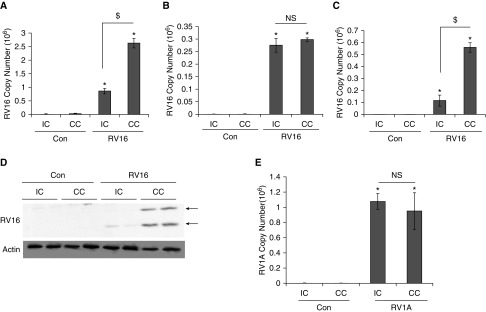
Although the airway epithelium is the primary site of RV infection, inflammatory cells such as monocytes and macrophages may also be important targets considering their high abundance in inflamed airways. In this study, we tried to understand the interaction between epithelial cells and monocytes by using a simple CC system in which both epithelial cells (A549) and monocytic cells (THP-1) were cocultivated in the same culture dish. We then separated epithelial cells (in adherent monolayer) and monocytes (in suspension) for analysis. After extensive washing, there were no monocytes left in the adherent epithelial culture, as confirmed by light microscopy. In the suspension, less than 0.1% of floating epithelial cells was usually detected by a differential cell count (data not shown). Thus, cross-contamination between the two cell types was negligible. Using this system, we found that the level of RV16 mRNA in monocytes was significantly higher in CC than in individual culture (IC) (Figure 1A). In contrast, RV16 mRNA abundance in epithelial cells was not significantly different between these two cultures (Figure 1B). Interestingly, under this CC condition, almost 10 times more RV RNA was detected in monocytes (Figure 1A) than in epithelial cells (Figure 1B), suggesting that either more viruses entered monocytes in CC or more active viral replication occurred in CC than in IC. Because RV16 is a positive-stranded RNA virus, the cellular level of negative strands was usually indicative of viral replication. Indeed, the abundance of negative strands of RV16 was also markedly increased in CC compared with IC (Figure 1C). Additionally, the levels of viral coat proteins (e.g., VP1 and VP4) were markedly increased in CC but barely detectable in IC, demonstrating a more robust translation in the CC (Figure 1D). Thus, a greater RV16 replication was most likely present in CC than in IC. Interestingly, there was no difference in the production of RV1A (a minor-group RV) between CC and IC in either monocytes (Figure 1E) or epithelial cells (data not shown).
Figure 1.
Replication of RV16 was significantly enhanced in monocytes when it was cocultivated with airway epithelial cells. THP-1 cells were cocultivated with A549 cells for 24 h. Then, RV16 or RV1A was added to the coculture (CC) for 24 h. Cells were separated and extensively washed as described in Materials and Methods before they were harvested. (A) RV16 mRNA was measured by quantitative PCR (qPCR) analysis in THP-1 cells. (B) RV16 mRNA was measured by qPCR analysis in A549 cells. (C) RV16-negative strand RNA was measured by qPCR analysis in THP-1 cells. Arrows represent viral coat proteins: VP0 (upper band) and VP4 (lower band). (D) RV coat proteins were analyzed by Western blot analysis. Actin was used as a loading control. Biological duplicates are shown. (E) RV1A mRNA was measured by qPCR analysis in A549 cells in both individual culture (IC) and CC when infected with RV1A. Triplicates were used for each experiment, and experiments were repeated at least three times. Data shown are mean ± SD. *P < 0.05 RV versus control; $P < 0.05 IC versus CC. Con, control treated with PBS (diluent of RV); NS, not significant; RV, human rhinovirus.
CM from Epithelial Cells Were Able to Enhance RV16 Replication
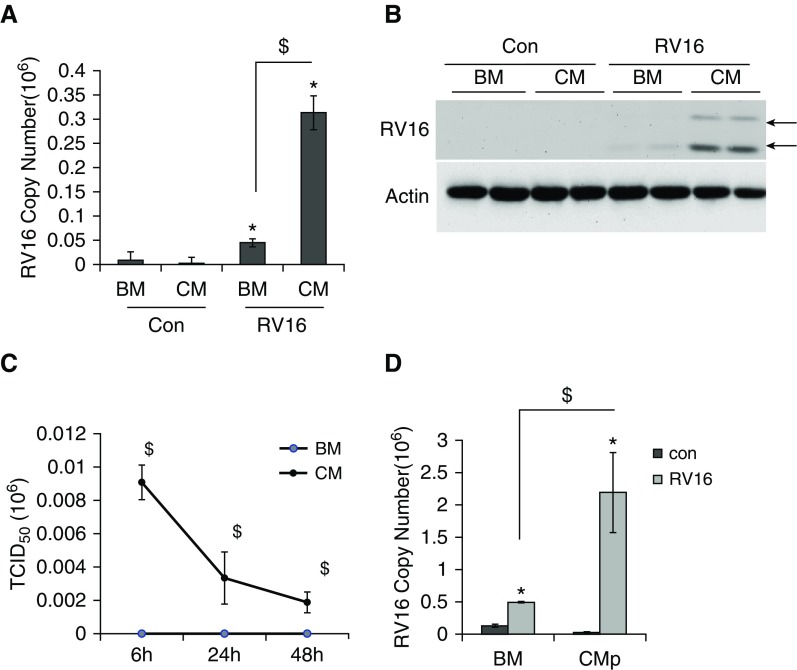
Based on the aforementioned results from the CC system, we reasoned that epithelial factors in CC media might be responsible for the increase of RV16 production in monocytes. Thus, we tested the effect of A549 CM on RV16 production in monocytes. Indeed, the CM treatment recapitulated the CC effect by significantly enhancing RV16 mRNA (Figure 2A) and coat protein (Figure 2B). Most importantly, a substantial amount of infectious RV16 was detected by TCID50 assay from the CM-treated cells, but not from the base medium (BM)-treated cells (Figure 2C). The amount of infectious viruses decreased over the time after infection, indicative of limited rounds of viral replication. To further confirm that these RVs were made from the active production rather than the release of previous cell-bound viruses, we performed an immunofluorescence analysis. As shown in Figure E1 in the online supplement, RV16 coat protein was detected in the permeated cells (treated with Triton X-100, +Triton), but not in the nonpermeated cells (−Triton). In the high-magnification image (80×), RV16 was indeed located intracellularly, but not on the cell membrane. Thus, the CM were indeed able to direct active viral replication and production in monocytes. Considering the potential bias from one cell line (i.e., A549), we tested CM from primary HBE cells (CMp). CMp was also found to dramatically increase RV16 replication in monocytes (Figure 2D). Taken together, these findings indicate that secretory epithelial factors stimulate active RV16 replication and production in monocytes.
Figure 2.
RV16 replication was significantly enhanced in monocytes in the presence of CM from epithelial cells. In the presence and absence of CM, monocytes were infected with RV16 as indicated and were then harvested for analysis after thorough washing with PBS. (A) RV16 mRNA was measured by qPCR analysis in THP-1 cells after RV infection for 24 h. (B) RV16 coat proteins were analyzed by Western blot analysis. Actin was used as a loading control. Biological duplicates are shown. Arrows represent viral coat proteins: VP0 (upper band) and VP4 (lower band). (C) Cells were infected for 24 h and thoroughly washed with PBS. Then, media were collected at 6 h, 24 h, and 48 h. Infectious viral particles were measured using a modified 50% tissue culture infective dose (TCID50) assay. (D) RV16 mRNA was measured by qPCR analysis in THP-1 cells in the presence of CM from primary HBE cells for 24 h. *P < 0.05 RV versus control; $P < 0.05 CM (or CMp) versus BM. BM, base media; CM, conditioned media from A549 cells; CMp, conditioned media from primary HBE cells; HBE, human bronchial epithelial.
RV Replication Significantly Enhanced Cytokine Expression in CM-Treated Monocytes
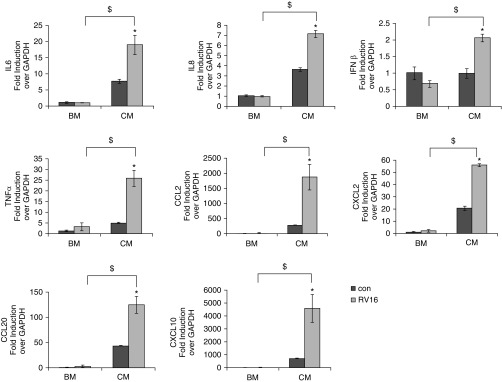
To further understand the biological effect of CM-induced RV16 replication, we measured the expression of cytokines in CM-treated and BM-treated monocytes. We found that a number of cytokines (i.e., IL-6, IL-8, IFN-β, TNF-α, CCL2, CXCL2, CCL20, and CXCL10) were significantly augmented by RV16 infection in CM-treated cells, but not in BM-treated cells (Figure 3). Additionally, as shown in Figure E2, UV-irradiated RV16 had no effect on the expression of IL-6, IL-8, IFN-β, TNF-α, or CCL2. It slightly increased CXCL2 and CCL20, but decreased CXCL10 in CM-treated cells. Notably, the basal expression level of these cytokines was very low to begin with. Thus, slight changes in the expression of CXCL2, CCL20, and CXCL10 might merely reflect random fluctuations when the expression levels were low. Nonetheless, these observations suggest that viral replication was indispensable for significantly inducing cytokine expression by RV16 infection of CM-treated cells.
Figure 3.
RV16 replication in monocytes significantly induced cytokine expressions. In the presence (CM) or absence (BM) of CM, monocytes were infected with RV16 and samples were harvested as described in Figure 2. RNA was isolated, followed by qPCR analysis. *P < 0.05 RV versus control; $P < 0.05 CM versus BM. CCL, chemokine (C–C motif) ligand; CXCL, chemokine (C–X–C motif) ligand.
ICAM1, the Receptor for Major-Group RVs, Was Significantly Elevated on Monocytes by CM Treatment
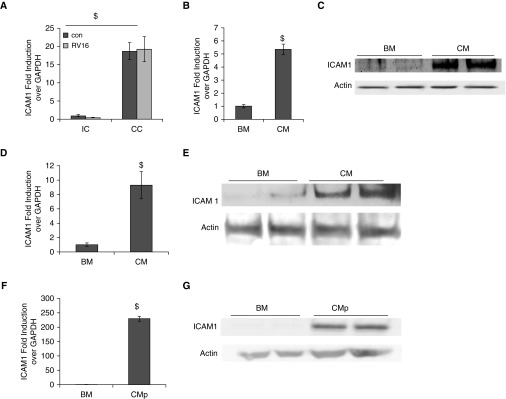
The lack of enhancement of RV1A (a minor-group RV) replication in cocultivated monocytes (Figure 1E) suggests that ICAM1, the receptor for major-group RVs, may play a role in this process. Indeed, we found that ICAM1 was highly elevated in monocytes when cocultivated with epithelial cells in the presence or absence of RV16 infection (Figure 4A). As expected, CM treatment also stimulated ICAM1 expression at both the mRNA (Figure 4B) and protein (Figure 4C) levels. To rule out any experimental bias caused by using one monocytic cell line, we tested primary PBMCs, and found that CM also highly elevated ICAM1 expression in these cells at both the mRNA (Figure 4D) and protein (Figure 4E) levels. Another potential cause of bias was our reliance on the CM from A549, a cancerous cell line. Thus, we further tested CM from primary HBE cells (CMp), and found that CMp were also able to significantly increase ICAM1 expression on THP-1 cells at both the mRNA (Figure 4F) and protein (Figure 4G) levels. Thus, a markedly increased ICAM1 expression appeared to be a common monocytic response to the CC condition or to CM from epithelial cells. Although what component(s) in the CM increased ICAM1 is unknown, it appeared to be heat resistant, as its stimulating effect was not abolished by heat inactivation (Figure E3).
Figure 4.
ICAM1 was highly elevated in both cocultivated and CM-treated monocytes. (A) THP-1 cells were cocultivated with A549 cells, and samples were harvested as described in Figure 1. ICAM1 mRNA THP-1 cells were measured by qPCR analysis. (B) In the presence (CM) or absence (BM) of A549 CM, THP-1 cells were infected with RV16. ICAM1 mRNA was measured by qPCR analysis. (C) ICAM1 protein was measured by Western blot analysis. Actin was used as a loading control. Biological duplicates are shown. (D) Primary PBMC cells were treated with CM or BM, and ICAM1 mRNA expression was determined by qPCR. (E) ICAM1 protein in primary PBMC cells was measured by Western blot analysis. Actin was used as a loading control. Biological duplicates are shown. (F) CM from primary HBE cells (CMp) were used. ICAM1 mRNA in THP-1 cells was measured by qPCR analysis. (G) ICAM1 protein in THP-1 cells treated with CMp or BM were measured by Western blot analysis. $P < 0.05 CC versus IC, CM versus BM, or CMp versus BM. ICAM1, intercellular adhesion molecule 1.
Other ICAM1-Inducing Stimuli Were Able to Enhance RV16 Production
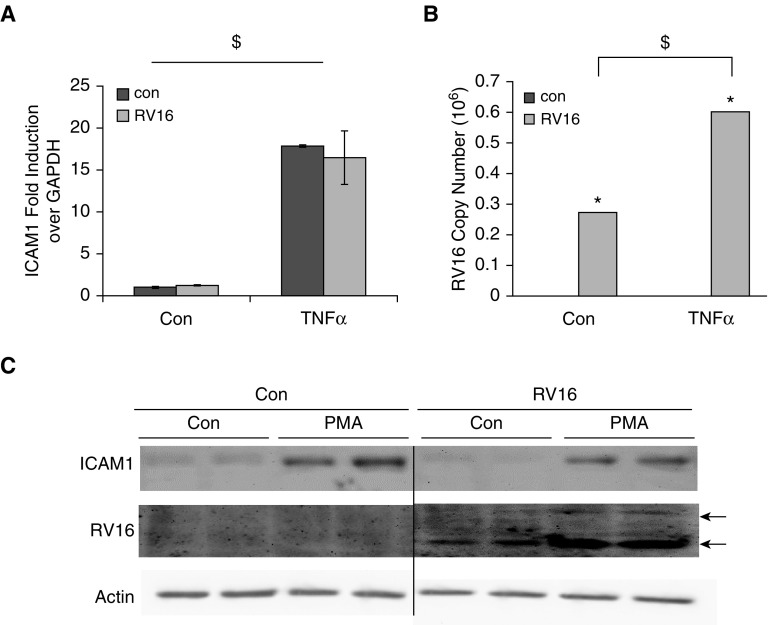
TNF-α was previously documented to increase ICAM1 expression (27). Thus, we sought to determine whether it could also increase RV16 production. Indeed, TNF-α treatment significantly increased ICAM1 expression (Figure 5A) independently of RV infection. It also enhanced the RV16 mRNA level as compared with nontreated cells (Figure 5B). Additionally, PMA is commonly used to induce differentiation from monocytes to macrophages. In a previous study using a PMA protocol, THP-1–derived macrophages were found to support RV16 replication (22). Interestingly, we found that PMA markedly increased ICAM1 expression and RV16 production (Figure 5C). Thus, the increase of ICAM1 expression and the enhancement of RV16 production appeared to be closely related.
Figure 5.
ICAM1-inducing stimuli significantly enhanced RV replication. (A) TNF-α treatment (24 h) enhanced ICAM1 expression in the presence or absence of RV infection in THP-1 cells. (B) RV mRNA was measured by qPCR analysis in THP-1 cells treated with TNF-α or left untreated in the absence or presence of RV infection. (C) Phorbol myristate acetate (PMA) treatment (72 h) increased ICAM1 expression in THP-1 cells, and RV production was measured by Western blot analysis. Actin was used as a loading control. Arrows represent viral coat proteins: VP0 (upper band) and VP4 (lower band). *P < 0.05 RV versus control; $P < 0.05 TNF-α versus nontreatment.
ICAM1 Was Required for RV Production in Monocytes
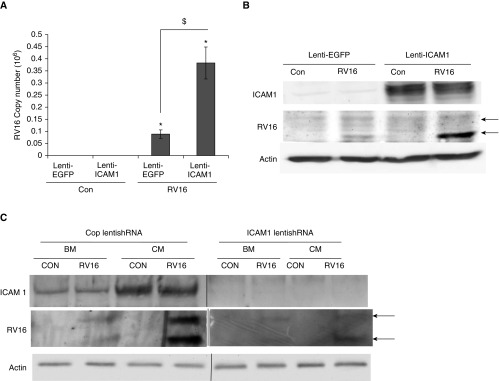
To conclusively demonstrate that ICAM1 overexpression in monocytes was indeed the cause of increased RV16 production, we ectopically overexpressed ICAM1 using a lentiviral transduction system. As described in Materials and Methods, an ICAM1 expression cassette was cloned into a lentiviral vector, and lentiviral particles containing ICAM1 were used to transduce monocytes. As shown in Figure 6B (top panel), lenti-ICAM1 successfully drove a significant ICAM1 expression in monocytes. When these cells were infected with RV16, viral mRNA abundance was much higher in lenti-ICAM1–transduced cells as compared with control cells infected with lenti-GFP (lentiviral particles containing a green fluorescence protein expression cassette; Figure 6A). Consistently, a much higher level of viral coat protein was also detected in these cells (middle panel, Figure 6B; a quantification of Figure 6B is provided in Figure E4). To further confirm the requirement of ICAM1 for RV infection in monocytes, we knocked down ICAM1 expression using lentiviral shRNA transduction. As shown in Figure 6C (top panel), lenti-shICAM1 dramatically reduced ICAM1 expression. Consistently, these monocytes with ICAM1 knocked down (lenti-shICAM1) were more resistant to RV infection than the control group (lenti-shC) (middle panel, Figure 6C). Taken together, these observations indicate that ICAM1 is both sufficient and necessary for active RV production in monocytes.
Figure 6.
Ectopic expression of ICAM1 enhanced RV production. (A) THP-1 cells were transduced with lenti-ICAM1 before they were infected with RV16. Lenti-enhanced green fluorescent protein (EGFP) virus was used as a negative control. RV mRNA was measured by qPCR analysis. *P < 0.05 RV versus control; $P < 0.05 lenti-ICAM1– versus lenti-EGFR–transduced cells. (B) RV16 coat protein or ICAM1 was measured by Western blot analysis. Actin was used as a loading control. (C) THP-1 cells were transduced with lenti-shRNA against ICAM1 (lenti-shICAM1) or control shRNA (lenti-shC) before they were infected with RV16. RV16 coat protein and ICAM1 were measured by Western blot analysis. Actin was used as a loading control. Arrows represent viral coat proteins: VP0 (upper band) and VP4 (lower band). shRNA, short hairpin RNA.
Discussion
Most studies on RV infection in human airways have focused on the airway epithelium. RV can replicate in epithelial cells and modulate their gene expression, thereby further influencing downstream events that ultimately lead to asthma and its exacerbation. The attention of the RV research community is increasingly being drawn to inflammatory cells. Because inflammation is a common phenotype associated with RV-induced illnesses, it is important to understand the RV-induced responses of inflammatory cells in inflamed airways and how they shape the progress of underlying disease. RV-induced airway inflammation was recapitulated in mouse models of RV1B infection (28, 29). RV1B, a minor-group RV, was shown to induce eotaxin-1 (28), IL-4 (28), IL-13 (28, 30), CCL2 (31), TNF-α (32, 33), CXCL1 (32), IL-6 (32), IFN-γ (33), and IL-12p40 (33) in mouse macrophages in the absence of viral replication or even RV internalization (32). Along this line, previous studies reported that a new pattern recognition receptor (PRR), Toll-like receptor 2 (TLR2), recognized nonreplicating RV particles and initiated a downstream inflammatory response by mouse monocytic cells (32, 34). However, no RV-specific, pathogen-associated molecular pattern that bound and activated TLR2 was identified. Thus, it is still unclear whether TLR2 directly sensed RV1B infection or the initial sensing was mediated by other, unknown PRRs. Because mice and mouse cells are nonpermissive to major-group RV infection, how human monocytic cells sense this group of RVs (e.g., RV16) is entirely unknown.
RV16 replication and its role in the induction of TNF-α production were reported in a study of monocyte-derived macrophages using a PMA protocol (22). In contrast, the parental monocytes had either no replication (i.e., in PBMC) or a very low grade of replication (i.e., in THP-1) (22). It was unclear what mechanism was activated by PMA to render naive monocytes permissive to RV. In the study presented here, we have demonstrated that RV16 entered and replicated in human monocytes when it was cocultivated with airway epithelial cells or exposed to epithelial cell CM. Most importantly, we showed that ICAM1 expression was highly elevated in monocytes cocultivated with epithelial cells or treated with CM from epithelial cells. Additionally, the PMA induction protocol used in the previous report (22) also significantly increased ICAM1 expression, suggesting a similar underlying mechanism. Lastly, overexpression or knockdown of ICAM1 confirmed the causal role of ICAM1 in mediating RV16 replication in monocytes. Thus, significantly enhanced ICAM1 expression appears to be responsible for the change in the permissiveness of monocytes to RV infection.
ICAM1 is the receptor for major-group RVs. In the solution, one RV was found to bind ∼60 soluble ICAM1s, each ICAM1 binding to one icosahedral face of the viral capsid. A previous study (35) shows that, in solution (no cells involved), one viral particle had to bind to multiple ICAM1 molecules in order to initiate uncoating and release of RV genome. In the cell culture, each RV16 was found to bind about five ICAM1 on the surface of HeLa cells (Dr. Waiming Lee, unpublished data). Thus, when the expression of ICAM1 on the cell surface is low, the probability that one RV will simultaneously bind multiple ICAM1s is also low, leading to the nonpermissive state for RV infection. Reciprocally, a high level of ICAM1 expression may induce a permissive state for RV infection. Indeed, we have shown that the enhancement of ICAM expression by multiple approaches (CC, CM, chemical treatment, or ectopic overexpression) could significantly stimulate RV replication in monocytes. Active viral replication implies that double-stranded RNA, a viral replicative intermediate and a well-studied pathogen-associated molecular pattern recognized by epithelial PRRs, may also be sensed by PRR(s) in human monocytes and trigger cellular IFN responses to repress RV replication and production (11, 36, 37). Thus, the infection of major-group RVs appears to be restrained by two cellular events: ICAM1-mediated viral entry and IFN-mediated antiviral defense. So far, there is no evidence supporting the replication of minor-group RVs in monocytic cells. However, a majority of the studies on minor-group RVs were done in mice or mouse cells. The mouse is not the natural host of RV and cannot support active RV replication. Thus, it is still unclear whether the lack of replication of minor-group RVs in mouse cells reflects a true nonpermissiveness of monocytic cells or a nonpermissiveness of mouse cells in general. Further studies on the infection of human monocytic cells by minor-group RVs are ongoing.
Monocytes are abundant in the lower airway of patients with respiratory illnesses. CC with epithelial cells was reported to induce the differentiation of monocytes into macrophage-like cells (38, 39). Interestingly, an increase of ICAM1 expression was shown to be an early marker indicative of monocyte-to-macrophage differentiation (40). Although ICAM1 was sufficient and necessary to mediate the change of permissiveness of monocytes in our study, the interaction between monocytes and epithelial cells (or CM from epithelial cells) was also likely to induce their differentiation to macrophages with the capacity to support RV replication (22). Thus, whether or not factors other than ICAM1 were induced during this process, and whether or not they were also involved in RV replication remain to be determined. Although the specific epithelial factor(s) that is responsible for the change in the permissiveness of monocytes to RV infection has not been discovered, it is known that ICAM1 expression can be activated by an NF-κB–dependent mechanism (41). Several cytokines (e.g., IL-6, IL-8, and CCL2) are abundantly secreted by epithelial cells and were shown to induce NF-κB activation (42–44). Therefore, further studies will be needed to test these candidates.
In summary, we have demonstrated, for the first time, that epithelial secretions direct robust RV replication in monocytes via significantly increased ICAM1. This new information will advance our understanding of the interaction between the airway epithelium and inflammatory cells in the context of RV infection and RV-induced disease exacerbation.
Footnotes
This work was supported by National Institutes of Health grants AI061695 and AI113526, grant 123055_CIA from the Flight Attendant Medical Research Institute, and Biomedical Investigator Award BIG-3064 from the Arizona Biomedical Research Commission.
Author Contributions: Y.C., X.Z., L.Z., and R.L. conceived or designed the study and acquired, analyzed, or interpreted data. Y.C. and X.Z. drafted the manuscript and revised it critically for important intellectual content, approved the final version for publication, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0271OC on March 22, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Savolainen C, Blomqvist S, Hovi T. Human rhinoviruses. Paediatr Respir Rev. 2003;4:91–98. doi: 10.1016/s1526-0542(03)00030-7. [DOI] [PubMed] [Google Scholar]
- 2.Gern JE. The ABCs of rhinoviruses, wheezing, and asthma. J Virol. 2010;84:7418–7426. doi: 10.1128/JVI.02290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotaniemi-Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy—the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. quiz 1188–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, Teague WG. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–321. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, Schnurr D, Ganem D, DeRisi JL, Boushey HA. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, Erwin EA, Shaker MS, Hellems M, Peerzada J, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venarske DL, Busse WW, Griffin MR, Gebretsadik T, Shintani AK, Minton PA, Peebles RS, Hamilton R, Weisshaar E, Vrtis R, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193:1536–1543. doi: 10.1086/503809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terajima M, Yamaya M, Sekizawa K, Okinaga S, Suzuki T, Yamada N, Nakayama K, Ohrui T, Oshima T, Numazaki Y, et al. Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1β. Am J Physiol. 1997;273:L749–L759. doi: 10.1152/ajplung.1997.273.4.L749. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Hamati E, Lee PK, Lee WM, Wachi S, Schnurr D, Yagi S, Dolganov G, Boushey H, Avila P, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol. 2006;34:192–203. doi: 10.1165/rcmb.2004-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaya M, Sekizawa K, Suzuki T, Yamada N, Furukawa M, Ishizuka S, Nakayama K, Terajima M, Numazaki Y, Sasaki H. Infection of human respiratory submucosal glands with rhinovirus: effects on cytokine and ICAM-1 production. Am J Physiol. 1999;277:L362–L371. doi: 10.1152/ajplung.1999.277.2.L362. [DOI] [PubMed] [Google Scholar]
- 13.Tyrrell DA, Mika-Johnson M, Phillips G, Douglas WH, Chapple PJ. Infection of cultured human type II pneumonocytes with certain respiratory viruses. Infect Immun. 1979;26:621–629. doi: 10.1128/iai.26.2.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gern JE, Dick EC, Lee WM, Murray S, Meyer K, Handzel ZT, Busse WW. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621–627. [PubMed] [Google Scholar]
- 15.Patel JA, Nair S, Ochoa EE, Huda R, Roberts NJ, Chonmaitree T. Interleukin-6-174 and tumor necrosis factor α-308 polymorphisms enhance cytokine production by human macrophages exposed to respiratory viruses. J Interferon Cytokine Res. 2010;30:917–921. doi: 10.1089/jir.2010.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston SL, Papi A, Monick MM, Hunninghake GW. Rhinoviruses induce interleukin-8 mRNA and protein production in human monocytes. J Infect Dis. 1997;175:323–329. doi: 10.1093/infdis/175.2.323. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber MT, Schuler B, Li L, Hall DJ. Activation of the small G-protein Rac by human rhinovirus attenuates the TLR3/IFN-α axis while promoting CCL2 release in human monocyte-lineage cells. Innate Immun. 2013;19:278–289. doi: 10.1177/1753425912460709. [DOI] [PubMed] [Google Scholar]
- 18.Hall DJ, Bates ME, Guar L, Cronan M, Korpi N, Bertics PJ. The role of p38 MAPK in rhinovirus-induced monocyte chemoattractant protein-1 production by monocytic-lineage cells. J Immunol. 2005;174:8056–8063. doi: 10.4049/jimmunol.174.12.8056. [DOI] [PubMed] [Google Scholar]
- 19.Karta MR, Wickert LE, Curran CS, Gavala ML, Denlinger LC, Gern JE, Bertics PJ. Allergen challenge in vivo alters rhinovirus-induced chemokine secretion from human airway macrophages. J Allergy Clin Immunol. 2014;133:1227–1230. doi: 10.1016/j.jaci.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korpi-Steiner NL, Bates ME, Lee WM, Hall DJ, Bertics PJ. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J Leukoc Biol. 2006;80:1364–1374. doi: 10.1189/jlb.0606412. [DOI] [PubMed] [Google Scholar]
- 21.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL. Respiratory virus induction of α-, β- and λ-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–386. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 22.Laza-Stanca V, Stanciu LA, Message SD, Edwards MR, Gern JE, Johnston SL. Rhinovirus replication in human macrophages induces NF-κB-dependent tumor necrosis factor α production. J Virol. 2006;80:8248–8258. doi: 10.1128/JVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Lee WM, Mosser AG, Rueckert RR. WIN 52035-dependent human rhinovirus 16: assembly deficiency caused by mutations near the canyon surface. J Virol. 1998;72:1210–1218. doi: 10.1128/jvi.72.2.1210-1218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oslund KL, Zhou X, Lee B, Zhu L, Duong T, Shih R, Baumgarth N, Hung LY, Wu R, Chen Y. Synergistic up-regulation of CXCL10 by virus and IFN γ in human airway epithelial cells. PLoS One. 2014;9:e100978. doi: 10.1371/journal.pone.0100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boisvert J, He X-S, Cheung R, Keeffe EB, Wright T, Greenberg HB. Quantitative analysis of hepatitis C virus in peripheral blood and liver: replication detected only in liver. J Infect Dis. 2001;184:827–835. doi: 10.1086/323391. [DOI] [PubMed] [Google Scholar]
- 26.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 27.Hubbard AK, Giardina C. Regulation of ICAM-1 expression in mouse macrophages. Inflammation. 2000;24:115–125. doi: 10.1023/a:1007029409521. [DOI] [PubMed] [Google Scholar]
- 28.Nagarkar DR, Bowman ER, Schneider D, Wang Q, Shim J, Zhao Y, Linn MJ, McHenry CL, Gosangi B, Bentley JK, et al. Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J Immunol. 2010;185:2525–2535. doi: 10.4049/jimmunol.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, et al. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med. 2008;177:1111–1121. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung Y, Hong JY, Lei J, Chen Q, Bentley JK, Hershenson MB. Rhinovirus infection induces interleukin-13 production from CD11b-positive, M2-polarized exudative macrophages. Am J Respir Cell Mol Biol. 2015;52:205–216. doi: 10.1165/rcmb.2014-0068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider D, Hong JY, Bowman ER, Chung Y, Nagarkar DR, McHenry CL, Goldsmith AM, Bentley JK, Lewis TC, Hershenson MB. Macrophage/epithelial cell CCL2 contributes to rhinovirus-induced hyperresponsiveness and inflammation in a mouse model of allergic airways disease. Am J Physiol Lung Cell Mol Physiol. 2013;304:L162–L169. doi: 10.1152/ajplung.00182.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saba TG, Chung Y, Hong JY, Sajjan US, Bentley JK, Hershenson MB. Rhinovirus-induced macrophage cytokine expression does not require endocytosis or replication. Am J Respir Cell Mol Biol. 2014;50:974–984. doi: 10.1165/rcmb.2013-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong JY, Chung Y, Steenrod J, Chen Q, Lei J, Comstock AT, Goldsmith AM, Bentley JK, Sajjan US, Hershenson MB. Macrophage activation state determines the response to rhinovirus infection in a mouse model of allergic asthma. Respir Res. 2014;15:63. doi: 10.1186/1465-9921-15-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han M, Chung Y, Young Hong J, Rajput C, Lei J, Hinde JL, Chen Q, Weng SP, Bentley JK, Hershenson MB. Toll-like receptor 2-expressing macrophages are required and sufficient for rhinovirus-induced airway inflammation. J Allergy Clin Immunol. 2016;138:1619–1630. doi: 10.1016/j.jaci.2016.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoover-Litty H, Greve JM. Formation of rhinovirus-soluble ICAM-1 complexes and conformational changes in the virion. J Virol. 1993;67:390–397. doi: 10.1128/jvi.67.1.390-397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol. 2009;40:610–619. doi: 10.1165/rcmb.2008-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crabbé A, Sarker SF, Van Houdt R, Ott CM, Leys N, Cornelis P, Nickerson CA. Alveolar epithelium protects macrophages from quorum sensing-induced cytotoxicity in a three-dimensional co-culture model. Cell Microbiol. 2011;13:469–481. doi: 10.1111/j.1462-5822.2010.01548.x. [DOI] [PubMed] [Google Scholar]
- 39.Osuský R, Malik P, Aurora Y, Ryan SJ. Monocyte-macrophage differentiation induced by coculture of retinal pigment epithelium cells with monocytes. Ophthalmic Res. 1997;29:124–129. doi: 10.1159/000268006. [DOI] [PubMed] [Google Scholar]
- 40.Coccia EM, Del Russo N, Stellacci E, Testa U, Marziali G, Battistini A. STAT1 activation during monocyte to macrophage maturation: role of adhesion molecules. Int Immunol. 1999;11:1075–1083. doi: 10.1093/intimm/11.7.1075. [DOI] [PubMed] [Google Scholar]
- 41.Melotti P, Nicolis E, Tamanini A, Rolfini R, Pavirani A, Cabrini G. Activation of NF-kB mediates ICAM-1 induction in respiratory cells exposed to an adenovirus-derived vector. Gene Ther. 2001;8:1436–1442. doi: 10.1038/sj.gt.3301533. [DOI] [PubMed] [Google Scholar]
- 42.Manna SK, Ramesh GT. Interleukin-8 induces nuclear transcription factor-κB through a TRAF6-dependent pathway. J Biol Chem. 2005;280:7010–7021. doi: 10.1074/jbc.M410994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 induces NF-κB activation in the intestinal epithelia. J Immunol. 2003;171:3194–3201. doi: 10.4049/jimmunol.171.6.3194. [DOI] [PubMed] [Google Scholar]
- 44.Viedt C, Dechend R, Fei J, Hänsch GM, Kreuzer J, Orth SR. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-κB and activating protein-1. J Am Soc Nephrol. 2002;13:1534–1547. doi: 10.1097/01.asn.0000015609.31253.7f. [DOI] [PubMed] [Google Scholar]