Abstract
Background & Aims
Little is known about the pathogenic mechanisms of gluten immunogenicity in patients with celiac disease. We studied temporal associations between infections and the development of celiac disease autoimmunity, and examined effects of HLA alleles, rotavirus vaccination status, and infant feeding.
Methods
We monitored 6327 children in the United States and Europe carrying HLA risk genotypes for celiac disease from 1 to 4 years of age for presence of tissue transglutaminase autoantibodies (the definition of celiac disease autoimmunity), until March 31, 2015. Parental reports of gastrointestinal and respiratory infections were collected every third month from birth. We analyzed time-varying relationships among reported infections, rotavirus vaccination status, time to first introduction of gluten, breastfeeding, and risk of celiac disease autoimmunity using proportional hazard models.
Results
We identified 13,881 gastrointestinal infectious episodes and 79,816 respiratory infectious episodes. During the follow-up period, 732 of 6327 (11.6%) children developed celiac disease autoimmunity. A GIE increased the risk of celiac disease autoimmunity within the following 3 months by 33% (hazard ratio [HR], 1.33; 95% confidence interval [CI], 1.11–1.59). This risk increased 2-fold among children born in winter and introduced to gluten before age 6 months (HR, 2.08; 95% CI, 1.46–2.98), and increased 10-fold among children without HLA-DQ2 alleles and breastfed for fewer than 4 months (HR, 9.76; 95% CI, 3.87–24.8). Risk of celiac disease autoimmunity was reduced in children vaccinated against rotavirus and introduced to gluten before age 6 months (HR, 0.57; 95% CI, 0.36-0.88).
Conclusions
Gastrointestinal infections increase the risk of celiac disease autoimmunity in children with genetic susceptibility to this autoimmune disorder. The risk is modified by HLA genotype, infant gluten consumption, breastfeeding, and rotavirus vaccination, indicating complex interactions among infections, genetic factors, and diet in the etiology of celiac disease in early childhood.
Keywords: Food, Gastroenteritis, Rotavirus, Autoimmunity
The role of childhood infections on the development of celiac disease is controversial. Although frequent infections during the first 18 months of life reportedly increase the risk of celiac disease later during childhood,1–5 acute infections at the time of gluten introduction have no effect on disease risk in the general population.6 This highlights the need for clarification if infections are associated with celiac disease in a large prospective multinational cohort. The Environmental Determinants of Diabetes in the Young (TEDDY) study recently showed country-specific differences in celiac disease risk during the first 2 years of life in genetically at-risk children.7 Because the TEDDY study prospectively collects clinical and environmental information on children starting at birth, association studies on infections before the development of disease are possible. The aim of this study was to characterize and determine temporal associations between gastrointestinal and respiratory infections on the subsequent risk of celiac disease autoimmunity (CDA) in children between 1 and 4 years of age.
Materials and Methods
Study Cohort
The TEDDY study, approved by local Ethical Institutional Review Boards and monitored by an External Advisory Board formed by the National Institutes of Health, follows up a birth cohort of 8676 children with 1 of 10 HLA-DR-DQ genotypes associated with type 1 diabetes and/or celiac disease,8 located in Finland; Germany; and Sweden; and Colorado, Georgia/Florida, and Washington state in the United States.9 Of the infants enrolled, 8280 (95.4%) carried one of the following HLA genotypes: DR3-DQ2/DR3-DQ2 (n = 1791), DR3-DQ2/DR4-DQ8 (n = 3339), DR4-DQ8/DR4-DQ8 (n = 1674), or DR4-DQ8/DR8-DQ4 (n = 1476). Clinical visits occurred every 3 months until 4 years of age and semiannually thereafter.10 Annual CDA screening began at age 2 years by measuring tissue transglutaminase antibody (tTGA) using a radioligand binding assay.7 Children who tested positive for tTGA at their annual visit were retested after 3 months, and those who persistently tested tTGA positive were defined as having CDA (Supplementary Figure 1). Previous quarterly serum samples were analyzed further to determine age at tTGA seroconversion. The present analysis included 6327 children who had reached 4 years of age by March 31,2015 (Supplementary Figure 2). Of those, 732 of 6327 (11.6%) developed CDA, of whom 318 underwent a duodenal biopsy and 283 (90.0%) had a biopsy indicative of celiac disease (ie, a Marsh score >1).
Reporting of Infectious Episodes and Rotavirus Vaccination
In TEDDY, caregivers continuously record a child's illnesses, hospitalizations, medical diagnoses, medication use, and vaccination history.8 For acute illnesses, caregivers record the symptoms, the date of the first appearance of symptoms, the diagnosis (if any), and the presence of any fever. Caregiver-reported symptoms and illnesses then are translated into International Classification of Diseases, 10th revision (ICD-10), codes by TEDDY staff during the scheduled clinical visits and categorized into respiratory, gastrointestinal, other, or unknown febrile infections.11 Other and unknown febrile infections were not considered in the current study because of low incidence.11 The respiratory and gastrointestinal infection categories included 11 and 2 medical diagnosis codes, respectively (Supplementary Table 1). ICD-10 codes for noninfective gastroenteritis (ie, K52, K52.0, K52.2, K52.8, and K52.9) were included as controls. To avoid overestimation of the number of total unique infections, any ICD-10 codes in the respiratory or gastrointestinal category recorded within 7 days were merged into one respiratory infectious episode (RIE) or gastrointestinal infectious episode (GIE), respectively.11
Statistical Methods
Age-specific RIE count was calculated as the total number of RIEs from the day after the previous visit until the end of the day of the next scheduled 3-month clinic visit. The cumulative count was calculated as a rate per year. Because incidence was lower, GIEs were analyzed as a binomial variable within any 1-year window of time and as a count per year over longer periods. The associations between RIE or GIE and CDA were examined by time-dependent Cox proportional hazards models. Children were right-censored after the day of the last negative CDA result or at 48 months of age, whichever came first. Children were left censored before 12 months of age. Two children with age of CDA seroconversion before 12 months were excluded. The current 3-month RIE/GIE rate was first examined for CDA risk. The time risk set was formed on the day of the first positive tTGA sample. The between-visit period over which the 3-month RIE/GIE rate was calculated for that specific day was considered the seroconversion period. The effect of RIE/GIE during the seroconversion period on CDA was assessed using hazard ratios (HRs) and 95% confidence intervals (CIs). RIE/GIE rates before the seroconversion period were examined as the cumulative RIE/GIE rate from enrollment, as the rate during the first year of life and last year before the seroconversion period, and as the rate between the 3-month visits within the first year of life and the last year before the seroconversion period (Supplementary Figure 1). All models were adjusted for potential CDA confounders or childhood infections (variables are listed in Table 1). Infections showing significant temporal association with CDA were tested further for interactions with CDA confounders as well as with season at the time of infection and age at seroconversion. Finally, the influence of country, rotavirus vaccination, and season on the odds of a GIE infection being reported between visits were examined by generalized estimating equations with logit link function. A P value less than .05 was considered statistically significant in primary analyses if the false discovery rate was less than .05. In all other analyses, a P value less than .05 was considered significant for description. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
Table 1. Proportional Hazards Model of Factors Associated With CDA or Rate of Childhood Infections on the Risk of CDA Between 1 and 4 Years.
| Total (n = 6327) | Univariate (n = 6327) | Multivariate (n = 6148) | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Country, n (%) | |||||
| United States | 2 545 (40.2) | 1 (reference) | 1 (reference) | ||
| Finland | 1 436 (22.7) | 1.09 (0.88–1.33) | 1.21 (0.97–1.50) | ||
| Germany | 338 (5.3) | 1.35 (0.97–1.89) | 1.35 (0.94–1.93) | ||
| Sweden | 2 008 (31.7) | 1.60 (1.35–1.89) | <.001 | 1.61 (1.31–1.97) | <.001 |
| Sex, n (%) | |||||
| Male | 3 253 (51.4) | 1 (reference) | 1 (reference) | ||
| Female | 3 074 (48.6) | 1.47 (1.27–1.71) | <.001 | 1.64 (1.41–1.90) | <.001 |
| HLA-DR-DQ genotype, n (%) | |||||
| DQ8/8 or DQ4/DR4-DQ8 | 2 385 (37.7) | 1 (reference) | |||
| DQ2/DQ8 | 2 581 (40.8) | 2.26 (1.81-2.82) | |||
| DQ2/DQ2 | 1 361 (21.5) | 6.31 (5.11-7.80) | <.001 | ||
| Per number of DQ2 haplotypes | 2.58 (2.33-2.85) | <.001 | 2.63 (2.37–2.92) | <.001 | |
| Season of birth, n (%) | |||||
| Summer (March–August) | 1 613 (49.4) | 1 (reference) | 1 (reference) | ||
| Winter (September–February) | 1 591 (50.6) | 0.86 (0.74-0.99) | .04 | 0.89 (0.77–1.03) | .12 |
| First-degree relative with celiac disease, n (%) | |||||
| No | 6 125 (96.8) | 1 (reference) | 1 (reference) | ||
| Yes | 202 (3.2) | 3.10 (2.40–4.02) | <.001 | 2.06 (1.58–2.69) | <.001 |
| Maternal age, y, median (IQR) | |||||
| For every 5-year increase in age | 31 (27–34) | 1.00 (0.93-1.07) | .92 | 0.92 (0.84–1.00) | .05 |
| Maternal education, n (%) | |||||
| High school | 1157 (18.7) | 1 (reference) | 1 (reference) | ||
| Trade school or some college | 1484 (24.0) | 1.05 (0.83-1.33) | 1.19 (0.92–1.53) | ||
| College degree or more | 3542 (57.3) | 1.22 (1.00-1.50) | .07 | 1.30 (1.03–1.63) | .03 |
| Only child in household at 9 months of age, n (%) | |||||
| No | 3576 (57.8) | 1 (reference) | 1 (reference) | ||
| Yes | 2608 (42.2) | 1.08 (0.93–1.25) | .31 | 1.05 (0.90–1.23) | .56 |
| Mode of delivery, n (%) | |||||
| Vaginal | 4709 (74.5) | 1 (reference) | 1 (reference) | ||
| Caesarian section | 1614 (25.5) | 0.78 (0.65–0.93) | .006 | 0.85 (0.71–1.03) | .09 |
| Age at start of daycare, n (%) | |||||
| <4 mo | 2312 (36.5) | 1 (reference) | |||
| 4 to <8 mo | 1292 (20.4) | 0.98 (0.81–1.18) | |||
| 8 to <12 mo | 683 (10.8) | 0.78 (0.60–1.01) | |||
| ≥12 mo | 2040 (32.2) | 0.76 (0.64–0.91) | .01 | ||
| For every 4-month increase in age | 0.92 (0.86–0.98) | .009 | 0.94 (0.88–1.01) | .11 | |
| Duration of any breastfeeding, n (%) | |||||
| <4 mo | 1681 (26.6) | 1 (reference) | |||
| 4 to <8 mo | 1471 (23.2) | 1.44 (1.16–1.79) | |||
| 8 to <12 mo | 1679 (26.4) | 1.33 (1.07–1.64) | |||
| ≥12 mo | 1440 (22.8) | 1.45 (1.17–1.80) | .002 | ||
| For every 4-month increase in duration | 1.11 (1.03–1.19) | .004 | 1.12 (1.04–1.22) | .004 | |
| Age at introduction to gluten, n (%) | |||||
| ≤4 mo | 1226 (19.4) | 1 (reference) | |||
| 5 mo | 1436 (22.7) | 0.83 (0.67–1.02) | |||
| 6 mo | 1444 (22.8) | 0.71 (0.57–0.88) | |||
| ≥7 mo | 2220 (35.1) | 0.74 (0.61–0.88) | .006 | ||
| For every 4-month increase in age | 0.83 (0.70–0.97) | .02 | 0.91 (0.75–1.11) | .34 | |
IQR, interquartile range.
Results
Temporal Association Between Infectious Episodes and Risk of Celiac Disease Autoimmunity
Complete demographic and dietary information was available for 97.2% (6148 of 6327) of subjects (Table 1). A total of 13,881 GIEs and 79,816 RIEs were recorded during the study period. RIEs and GIEs peaked between 6 and 9 months of age (69.7% of children reporting) and 15 to 18 months of age (18.2% of children reporting), respectively (Supplementary Figure 3A). The incidence of RIEs and GIEs was highest in February (5.0 and 1.09 per person-years, respectively) and lowest in July (1.6 and 0.32 per person-years, respectively) (Supplementary Figure 3B). After adjusting for risk factors (Table 1), RIE and GIE rates reported either within the seroconversion period, cumulatively since enrollment, or during the first year of life were not associated with CDA (Figure 1). There was no CDA risk at any time after a recent RIE, after correcting for multiple comparisons (Figure 1B). However, a GIE occurring within any 3-month period significantly increased the CDA risk within the following 3 months (adjusted HR, 1.33; 95% CI, 1.11–1.59; P = .002; false discovery rate < 0.05) (Figure 1A). Both bacterial or viral infective gastroenteritis (adjusted HR, 1.33; 95% CI, 1.08-1.63) and nausea and vomiting (adjusted HR, 1.36; 95% CI, 1.12-1.22) medical diagnosis codes were associated with CDA in this time window, whereas noninfective gastroenteritis was not associated (adjusted HR, 1.06; 95% CI, 0.55-2.06) (Supplementary Table 2).
Figure 1.
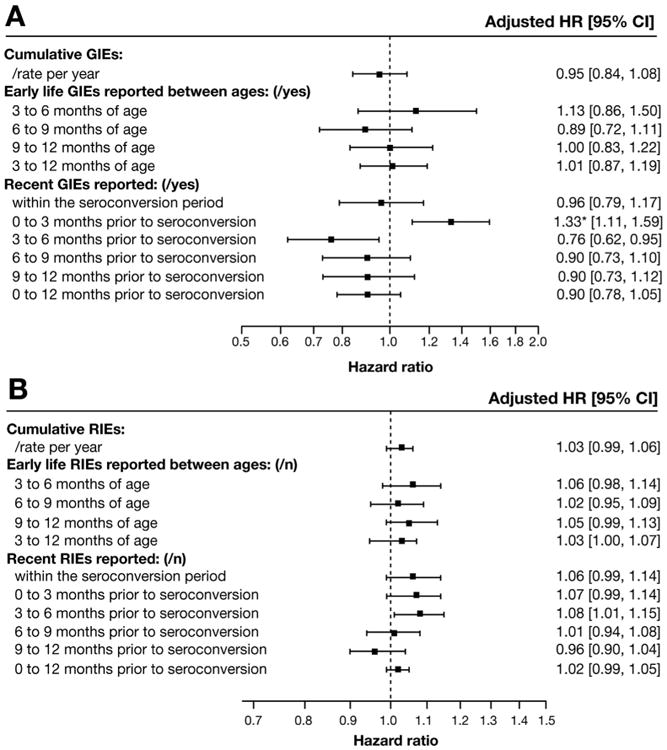
Risk of CDA between 1 and 4 years of age for (A) GIEs and (B) RIEs reported in the first year of life and within a year before the seroconversion period. *Statistically significant as P < .05 and false discovery rate less than 0.05.
Effect Modifications
Secondary analyses were conducted to further characterize the influence of GIEs occurring 0 to 3 months before the seroconversion period on CDA risk. The association between CDA and GIE 0 to 3 months before the seroconversion period was not modified by country (P = .46), sex (P = .89), HLA-DQ genotype (P = .18), season of gastrointestinal infection (P = .14), first-degree relative with celiac disease (P = .42), maternal age (P = .62), maternal education (P = .25), number of children in the household at 9 months of age (P = .13), mode of delivery (P = .46), age at start of daycare (P = .56), or age at introduction to gluten (P = .57). However, the association was modified by duration of any breastfeeding (2-way interaction, P = .03), HLA-DQ genotype (3-way interaction, P = .002), by season of birth (2-way interaction, P = .05), and by age at gluten introduction (3-way interaction, P < .001). An interaction between age at tTGA seroconversion (2-way interaction, P = .04) also was found. For children who were breastfeed for less than 4 months the influence of GIEs on CDA risk (adjusted HR, 2.02; 95% CI, 1.37-2.96) was stronger compared with those who were breastfed longer (Figure 2). This risk was even more pronounced among children carrying the HLA-DQ8/8 or HLA-DQ4/8 genotypes (adjusted HR, 9.76; 95% CI, 3.87–24.80) (Figure 2). Examining exclusive breastfeeding as a factor supported the findings of any breastfeeding on the association between GIE and CDA (data not shown).
Figure 2.
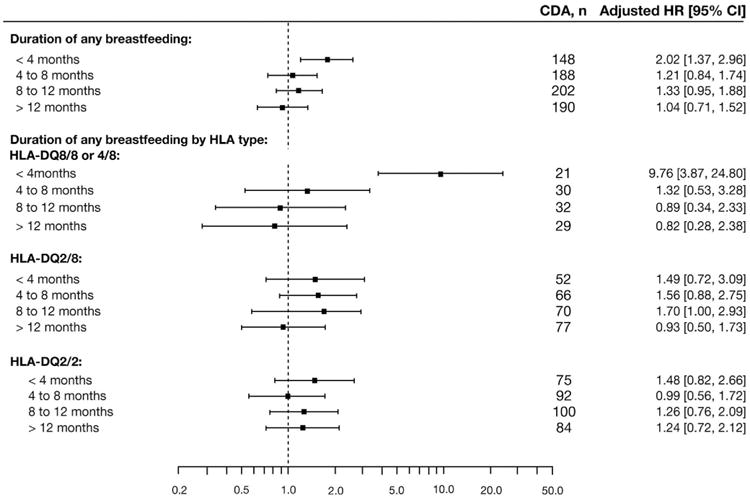
Stratified proportional hazards models of GIE 0 to 3 months before the seroconversion period on the risk of CDA between 1 and 4 years of age stratified by duration of any breastfeeding and HLA-DQ genotype.
The GIE-CDA association also was strengthened among children born in winter (born September–February: adjusted HR, 1.56; 95% CI, 1.21–2.02) compared with children born in summer (born March-August: adjusted HR, 1.11; 95% CI, 0.85–1.44) (Figure 3), and seemed to be strengthened further if gluten introduction occurred before 6 months of age (adjusted HR, 2.08; 95% CI, 1.46–2.98). There was no association if gluten was introduced at 6 months or later, or if a child born in summer was introduced to gluten before 6 months of age (Figure 3). However, the season of birth effect on the GIE-CDA association was seen only among Swedish children (interaction, P = .01) and not at other TEDDY sites (interaction, P = .96), because 69.7% (241 of 346) of CDA cases with early gluten introduction were Swedish. Swedish participants were introduced to gluten at least 1 month earlier than children from other TEDDY sites (Supplementary Table 3).
Figure 3.
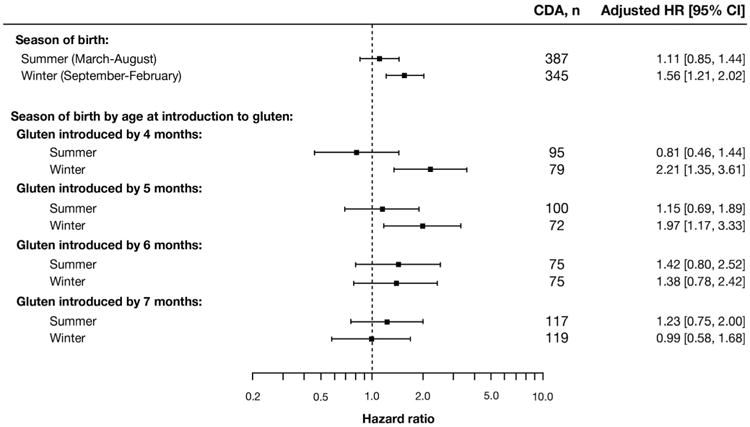
Stratified proportional hazards models of GIE 0 to 3 months before the seroconversion period on the risk of CDA between 1 and 4 years of age stratified by season of birth and age at introduction to gluten.
Rotavirus Vaccination and Risk of Celiac Disease Autoimmunity
Although country of birth did not appear to modify the GIE-CDA association overall (2-way interaction, P = .46), the association was similarly high in Sweden (HR, 1.45; 95% CI, 1.12–1.87) and Germany (HR, 1.56; 95% CI, 0.70-3.48), where the rotavirus vaccination coverage was low (Supplementary Figure 4), but not observed in Finland (HR, 0.99; 95% CI, 0.62–1.57) or in the United States (HR, 1.22; 95% CI, 0.86–1.73) where the rotavirus vaccination coverage was high. The rotavirus vaccine had a modifying effect on how GIE related to CDA for participants in Finland and the United States (2-way interaction, P < .05); a GIE within any 3-month period increased the risk of CDA within the following 3 months only in unvaccinated children (adjusted HR, 1.46; 95% CI, 1.03–2.09) and not in vaccinated children (adjusted HR, 0.81; 95% CI, 0.51–1.28). A look at the percentage of children with a GIE between the 3-month visits over time showed Sweden (OR, 1.71; 95% CI, 1.61-1.81), Germany (OR, 1.26; 95% CI, 1.12-1.42), as well as Finland (OR, 1.28; 95% CI, 1.20-1.36) as having significantly higher odds of having a GIE in a 3-month period compared with children in the United States (Figure 4A and B). For children in the United States and Finland, vaccination against rotavirus before 3 months of age, which covered 96% of all rotavirus-vaccinated children, was associated with an 8% lower odds of GIE between a visit (OR, 0.92; 95% CI, 0.86-0.98), and a 16% lower odds if the child was younger than 2 years of age (OR, 0.84; 95% CI, 0.76-0.92; age interaction, P < .001) (Figure 4C and D). Gluten introduction showed evidence of further modifying the association between rotavirus vaccination and CDA (interaction, P = .05) (Figure 5). After adjusting for all other confounding factors, children from Finland and the United States who were introduced to gluten before 6 months of age had a 43% reduced risk of CDA if they had received the rotavirus vaccine (adjusted HR, 0.57; 95% CI, 0.37-0.88).
Figure 4.
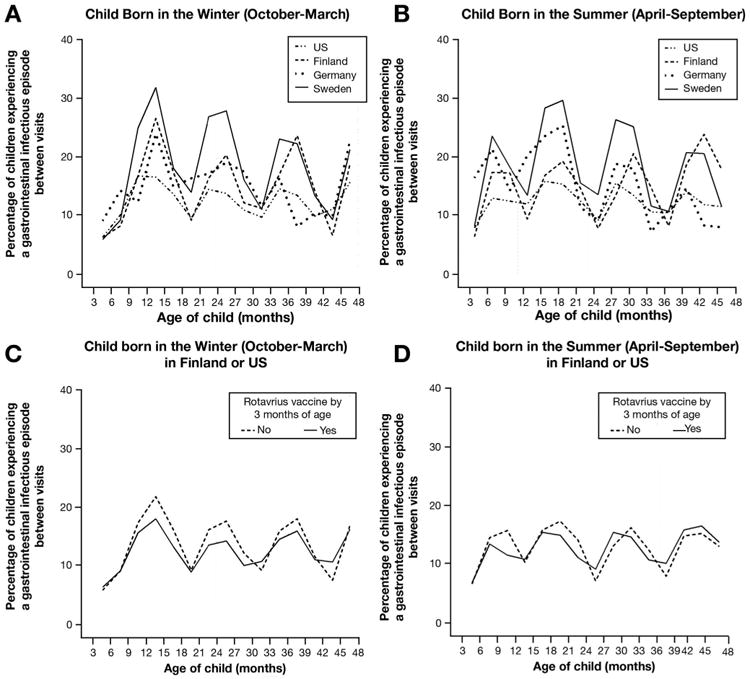
Proportion of TEDDY children reporting a gastrointestinal infection over time among those (A) born in winter or (B) born in summer, and in relation to rotavirus vaccination status in American and Finnish TEDDY children (C) born in winter or (D) born in summer.
Figure 5.
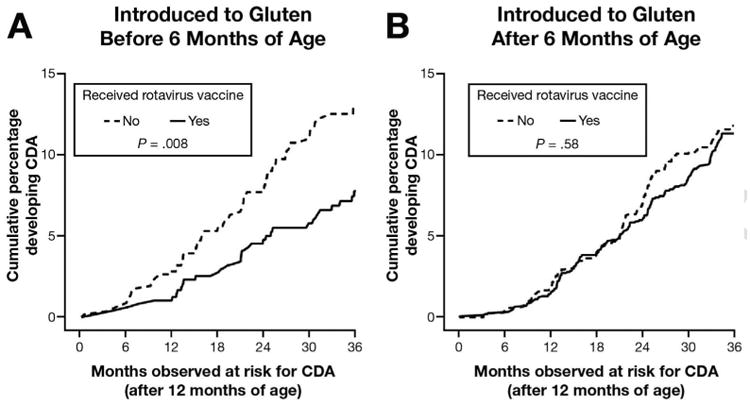
Kaplan–Meier curves and log-rank test of CDA by rotavirus vaccine separately for children introduced to gluten (A) before or (B) after 6 months of age.
Discussion
In TEDDY, gastrointestinal infections occurring 3 months before the seroconversion period may trigger CDA in children carrying HLA risk genotypes for celiac disease. The increased risk after a gastrointestinal infection is modified further by several early life factors including the duration of any breastfeeding, age of gluten introduction, season of birth, HLA genotype, and status of rotavirus vaccination, pointing to important interactions between diet and infections in the development of CDA during early childhood.
Most GIEs in TEDDY are thought to be of viral origin because their incidence peaks in winter (Supplementary Figure 3)11 and viral infections, specifically those by rota-, noro-, astro-, or adenoviruses, are also the most common cause of acute gastroenteritis in young children.12 Previous studies have suggested rotavirus as a trigger for celiac disease because early onset of CDA in children is correlated with serologic evidence of repeated rotavirus infections4 and tTGA-positive serum from active celiac disease patients recognizes peptides of the rotavirus outer capsid protein VP-713 Indeed, the protective effect of rotavirus vaccination in this study suggests that at least part of the associated gastrointestinal infections may be caused by rotavirus. Two rotavirus vaccines became available during the TEDDY enrollment period, leading to wide variability in vaccine coverage (Supplementary Figure 4).14-16 Although prior rotavirus vaccination was not protective of CDA in itself in Finland and the United States, vaccinated children from these countries who were introduced to gluten before 6 months of age had a 43% reduction in CDA risk. Thus, the timing between gluten exposure and rotavirus infection seems important in the development of CDA. Alternatively, the reduction in CDA risk in children introduced to gluten early may be a consequence of the live oral vaccine inducing tolerance to dietary antigens in the intestine.17 In either case, viruses causing gastroenteritis in young children should be studied further as possible CDA risk factors.
In addition, a GIE also greatly increased CDA risk in the following 3-month period in a small group of non--HLA-DQ2 risk carriers (ie, HLA-DQ8/8 or HLA-DQ4/8) who were breastfed for less than 4 months. Although breastfeeding duration itself is not a risk factor for celiac disease according to a recent meta-analysis,18 it contributes to passive immunity in infancy. Breast milk contains numerous antimicrobial compounds, including maternal secretory IgA that coats the respiratory and gastrointestinal mucosal surfaces to neutralize infectious agents for protection against diarrheal diseases.19 The pronounced risk among non-HLA-DQ2 risk carriers breastfed for fewer than 4 months suggests that the effect a GIE has on CDA risk is HLA-dependent. The gene dose effect of HLA-DQ2 on the risk of CDA is well described.7 Thus, it is interesting that a small group of TEDDY children carrying the non-HLA-DQ2 risk genotypes were most at risk for CDA after a GIE. A recent study found that the intestinal microbiome of 1-month-old breastfed non-HLA-DQ2/8 risk carriers was reduced in diversity and richness compared with HLA-DQ2 risk carriers.20 Coupled with reduced passive immunity owing to early cessation of breastfeeding,19 this can create a proinflammatory milieu in the intestine for gluten to trigger celiac disease after a GIE in non-HLA-DQ2 risk carriers. Early cessation of breastfeeding is correlated with early gluten introduction,21,22 making it difficult to separate these effects and suggests reasons for why both factors modify the association between GIE and CDA.
Although symptoms of gastroenteritis close to tTGA seroconversion could be misidentified as early signs of celiac disease, this study mitigates this possibility by the following: (1) analysis of infectious episodes rather than individual symptoms suggests the associated gastroenteritis is of infective nature; (2) no increase in CDA risk observed after a GIE in the seroconversion period, which would be expected if the associated GIE was simply a symptom of celiac disease; and (3) parent reported noninfective gastroenteritis symptoms (defined as gastrointestinal symptoms not suspected to be infectious) occurring within 3 months before the seroconversion period are not associated significantly with an increased risk of CDA.
A strength of TEDDY is the prospective collection of information before CDA onset, which minimizes recall bias in symptom reporting. Frequent sampling also minimizes the potential of reverse causation. Comprehensive data collection and large sample size allowed for sufficient power to perform temporal analysis stratified by numerous risk factors, which showed several important modifiers of the association between GIEs and CDA. A weakness is the possible impact of selection bias caused by under-representation of younger families and families from the United States and Germany.23 Although the drop-out rate was high before 2 years of age, compliance at blood draws and clinic visits was good (87.4%) in children who still were enrolled at 2 years of age. The self-reporting of illnesses was another limitation. The causative microbial agent was unspecified for most reported illnesses in TEDDY because even when an illness required consultation by a health care provider, laboratory analyses were not always performed if the infection could be treated empirically. However, self-reporting of symptoms does capture mild and self-limiting infections that may not require medical attention. The rates of infection in our study were similar to those reported among children of similar age in 2 population-based studies in Norway and Sweden,3,6 suggesting that this approach is appropriate. An increase in celiac disease risk was observed among Norwegian children affected by more multiple infections early in life.3 Those findings were confirmed here in a univariate model examining the CDA risk from any infection (Supplementary Table 4). However, after adjusting for country and other known risk factors, the association was no longer significant, suggesting that the previous may be Norway-specific, whereas, in TEDDY, a GIE increasing the CDA risk within the following 3-month period is a more generalizable phenomenon across the participating countries in TEDDY.
Conclusions
A gastrointestinal infection increases CDA risk in the following 3-month period in children at genetic risk for celiac disease. The timing of infection is important and its effect on CDA risk is modified further by early introduction to gluten in children born in winter, short duration of any breastfeeding in non-DQ2 risk carriers, and status of rotavirus vaccination. This indicates that various exposures during early childhood may trigger CDA and encourages further work on the role of viruses, especially rotavirus, as a trigger for CDA in early life.
Supplementary Material
Supplementary Figure 1. Example of sampling and lag periods in relation to tTGA seroconversion and CDA. In this example, the 24 and 27-month serum samples are confirmed positive for tTGA. Therefore the date of seroconversion is set at 24 months. The seroconversion period is the three-month interval between the last negative (21 month) and first positive (24 month) tTGA sample. tTGA, tissue transglutaminase autoantibody; CDA, celiac disease autoimmunity.
Supplementary Figure 2. Flow chart describing the cohort study population. HLA, human leukocyte antigen; tTGA, tissue transglutaminase autoantibody; CDA, celiac disease autoimmunity.
Supplementary Figure 3. RIEs and GIEs reported in the TEDDY study. (A) Proportion of children reporting a RIE or GIE within three-month age intervals. (B) Incidence of RIEs and GIEs reported by calendar month. RIE, respiratory infectious episode; GIE, gastrointestinal infectious episode.
Supplementary Figure 4. Rotavirus vaccine coverage at one year of age by country and year of birth. + A few subjects enrolled in 2004 are included in 2005, and a few subjects born in January and February 2010 are included in the 2009 count.
Supplementary Table 1. List of Medical Diagnosis Codes in the RIE and GIE Categories
Supplementary Table 2. Different Types of Reported Gastrointestinal Illnesses in the 3-Month Period Before the Seroconversion Period and Risk of CDA
Supplementary Table 3. Median Age at Gluten Introduction, by Country
Supplementary Table 4. Time-Dependent Proportional Hazards Model of Cumulative Infectious Episodes Between 3 and 18 Months of Age on the Risk of CDA Between 1 and 4 Years of Age With and Without Adjustment for Factors Associated With CDA
Acknowledgments
Funding: This study was funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4∼ DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, and UC4 DK106955 rants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and contract HHSN267200700014C from the National Institutes of Health (NIH), as well as the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and the Centers for Disease Control and Prevention (CDC). This work was supported in part by the National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
Abbreviations used in this paper
- CDA
celiac disease autoimmunity
- CI
confidence interval
- GIE
gastrointestinal infectious episode
- HR
hazard ratio
- ICD-10
International Classification of Diseases, 10th revision
- RIE
respiratory infectious episode
- TEDDY
The Environmental Determinants of Diabetes in the Young
- tTGA
tissue transglutaminase autoantibody
Footnotes
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2016.10.033.
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Myléus A, Hernell O, Gothefors L, et al. Early infections are associated with increased risk for celiac disease: an incident case-referent study. BMC Pediatr. 2012;12:194. doi: 10.1186/1471-2431-12-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canova C, Zabeo V, Pitter G, et al. Association of maternal education, early infections, and antibiotic use with celiac disease: a population-based birth cohort study in northeastern Italy. Am J Epidemiol. 2014;180:76–85. doi: 10.1093/aje/kwu101. [DOI] [PubMed] [Google Scholar]
- 3.Mårild K, Kahrs CR, Tapia G, et al. Infections and risk of celiac disease in childhood: a prospective nationwide cohort study. Am J Gastroenterol. 2015;110:1475–1484. doi: 10.1038/ajg.2015.287. [DOI] [PubMed] [Google Scholar]
- 4.Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101:2333–2340. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 5.Welander A, Tjernberg AR, Montgomery SM, et al. Infectious disease and risk of later celiac disease in childhood. Pediatrics. 2010;125:e530–e536. doi: 10.1542/peds.2009-1200. [DOI] [PubMed] [Google Scholar]
- 6.Sandberg-Bennich S, Dahlquist G, Källén B. Coeliac disease is associated with intrauterine growth and neonatal infections. Acta Paediatr. 2002;91:30–33. doi: 10.1080/080352502753457905. [DOI] [PubMed] [Google Scholar]
- 7.Liu E, Lee HSS, Aronsson CA, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:42–49. doi: 10.1056/NEJMoa1313977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagopian WA, Lernmark A, Rewers MJ, et al. TEDDY-The Environmental Determinants of Diabetes in the Young: an observational clinical trial. Ann N Y Acad Sci. 2006;1079:320–326. doi: 10.1196/annals.1375.049. [DOI] [PubMed] [Google Scholar]
- 9.Hagopian WA, Erlich H, Lernmark A, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12:733–743. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8:286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 11.Lönnrot M, Lynch K, Larsson HE, et al. A method for reporting and classifying acute infectious diseases in a prospective study of young children: TEDDY. BMC Pediatr. 2015;15:24. doi: 10.1186/s12887-015-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiegering V, Kaiser J, Tappe D, et al. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis. 2011;15:e401–e407. doi: 10.1016/j.ijid.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Zanoni G, Navone R, Lunardi C, et al. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006;3:e358. doi: 10.1371/journal.pmed.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widdowson MAA, Steele D, Vojdani J, et al. Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines. J Infect Dis. 2009;200(Suppl 1):S1–S8. doi: 10.1086/605061. [DOI] [PubMed] [Google Scholar]
- 15.Nohynek H, Salo H, Renko M, et al. Finland introduces rotavirus vaccine into the national vaccination programme in September 2009. Euro Surveill. 2009;14:35. [PubMed] [Google Scholar]
- 16.Uhlig U, Kostev K, Schuster V, et al. Rotavirus vaccination in Germany: analysis of nationwide surveillance data 2006 to 2010. Pediatr Infect Dis J. 2011;30:e244–e247. doi: 10.1097/INF.0b013e31822d1408. [DOI] [PubMed] [Google Scholar]
- 17.Poonam P. The biology of oral tolerance and issues related to oral vaccine design. Current Pharm Design. 2007;13:2001–2007. doi: 10.2174/138161207781039814. [DOI] [PubMed] [Google Scholar]
- 18.Szajewska H, Shamir R, Chmielewska A, et al. Systematic review with meta-analysis: early infant feeding and coeliac disease-update 2015. Aliment Pharmacol Ther. 2015;41:1038–1054. doi: 10.1111/apt.13163. [DOI] [PubMed] [Google Scholar]
- 19.Jackson KM, Nazar AM. Breastfeeding, the immune response, and long-term health. J Am Osteopath Assoc. 2006;106:203–207. [PubMed] [Google Scholar]
- 20.Olivares M, Neef A, Castillejo G, et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut. 2015;64:406–417. doi: 10.1136/gutjnl-2014-306931. [DOI] [PubMed] [Google Scholar]
- 21.Clayton HB, Li R, Perrine CG, et al. Prevalence and reasons for introducing infants early to solid foods: variations by milk feeding type. Pediatrics. 2013;131:e1108–e1114. doi: 10.1542/peds.2012-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klag EA, McNamara K, Geraghty SR, et al. Associations between breast milk feeding, introduction of solid foods, and weight gain in the first 12 months of life. Clin Pediatr. 2015;54:1059–1067. doi: 10.1177/0009922815569202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lernmark B, Lynch K, Baxter J, et al. Participant experiences in the Environmental Determinants of Diabetes in the Young study: common reasons for withdrawing. J Diabetes Res. 2016;2016:2720650. doi: 10.1155/2016/2720650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Example of sampling and lag periods in relation to tTGA seroconversion and CDA. In this example, the 24 and 27-month serum samples are confirmed positive for tTGA. Therefore the date of seroconversion is set at 24 months. The seroconversion period is the three-month interval between the last negative (21 month) and first positive (24 month) tTGA sample. tTGA, tissue transglutaminase autoantibody; CDA, celiac disease autoimmunity.
Supplementary Figure 2. Flow chart describing the cohort study population. HLA, human leukocyte antigen; tTGA, tissue transglutaminase autoantibody; CDA, celiac disease autoimmunity.
Supplementary Figure 3. RIEs and GIEs reported in the TEDDY study. (A) Proportion of children reporting a RIE or GIE within three-month age intervals. (B) Incidence of RIEs and GIEs reported by calendar month. RIE, respiratory infectious episode; GIE, gastrointestinal infectious episode.
Supplementary Figure 4. Rotavirus vaccine coverage at one year of age by country and year of birth. + A few subjects enrolled in 2004 are included in 2005, and a few subjects born in January and February 2010 are included in the 2009 count.
Supplementary Table 1. List of Medical Diagnosis Codes in the RIE and GIE Categories
Supplementary Table 2. Different Types of Reported Gastrointestinal Illnesses in the 3-Month Period Before the Seroconversion Period and Risk of CDA
Supplementary Table 3. Median Age at Gluten Introduction, by Country
Supplementary Table 4. Time-Dependent Proportional Hazards Model of Cumulative Infectious Episodes Between 3 and 18 Months of Age on the Risk of CDA Between 1 and 4 Years of Age With and Without Adjustment for Factors Associated With CDA


