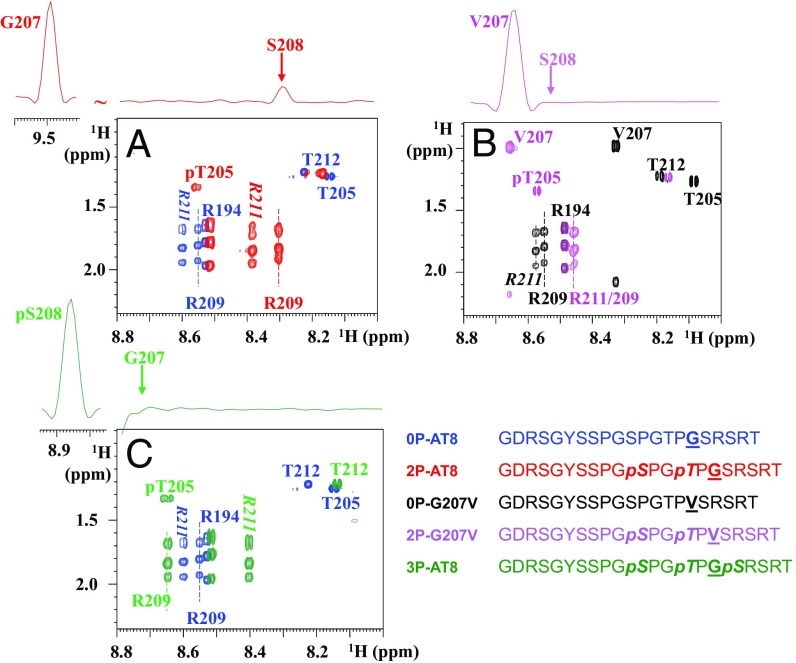
Fig. 1.
Breaking the turn-like structure by the Gly207Val mutation or by phosphorylation of Ser208. Zooms of the homonuclear TOCSY spectra of the nonmodified and phosphorylated peptides centered on the Thr205 and Arg209/211 residues. (Upper) The 1D traces are extracted from the corresponding NOESY spectra on the phosphorylated peptides. (A) Phosphorylation of the WT peptide at Ser202/Thr205, as in the 2P-AT8 peptide, leads to an important shift for the Arg209 amide proton and is accompanied by an NOE contact between the amide protons of Gly207 and Ser208, indicative of the phosphorylation-induced turn (14). (B) When Gly207 is substituted for a Val, the absence of an NOE contact in the 2P-G207V peptide between the amide protons of Val207 and Ser208 and the reduced shift for Arg209 indicate a destabilization of the turn-like structure. (C) Additional phosphorylation of Ser208 in the 3P-AT8 peptide has a similar effect as the Gly-to-Val substitution at position 207.