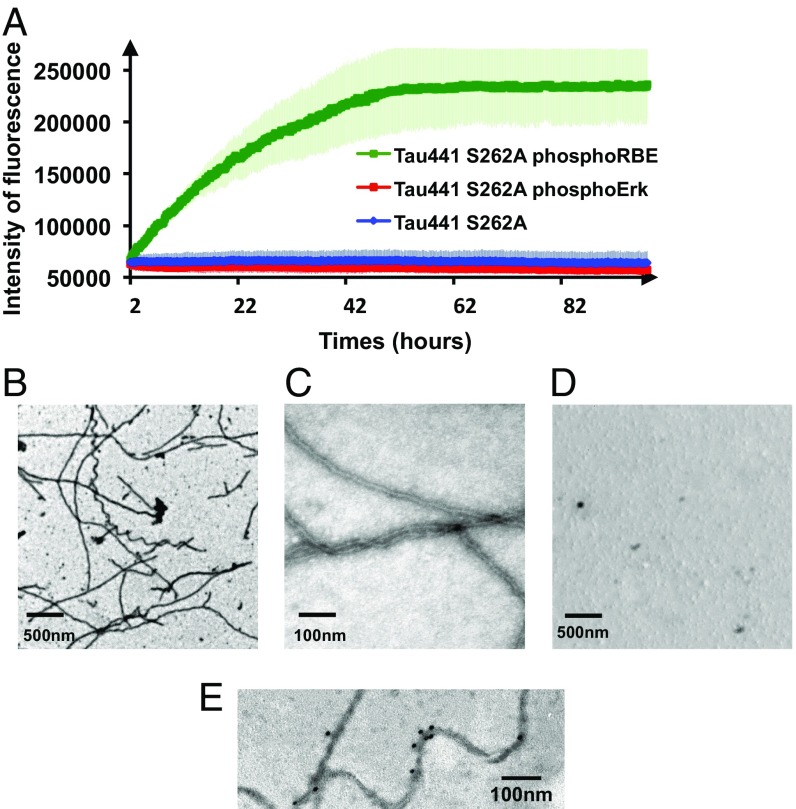
Fig. 4.
Additional phosphorylation of Ser208 by RBE promotes aggregation of Tau441-Ser262A (A) Aggregation of Tau441-S262A (blue), Tau441-S262A phosphorylated by ERK2 (red), and Tau441-S262A phosphorylated by RBE (green) followed by ThT emission at 490 nm. An increase in ThT emission at 490 nm is observed only for the 3P-AT8 Tau441-S262A protein. Error bars correspond to three independent aggregation experiments, with new batches of proteins and different RBEs. (B–D) TEM images at the end point of the aggregation assay of Tau441-S262A phosphorylated by RBE (B and C) or by Erk (D) confirm the results obtained in the aggregation assay. Large amounts of fibrils are observed only for Tau441-S262A phosphorylated by RBE. (E) Immunogold electron microscopy of the fibers obtained with Tau441-S262A phosphorylated by RBE. AT8 indeed stains the fibers in a similar manner as AD brain-derived fibers.