Figure 2.
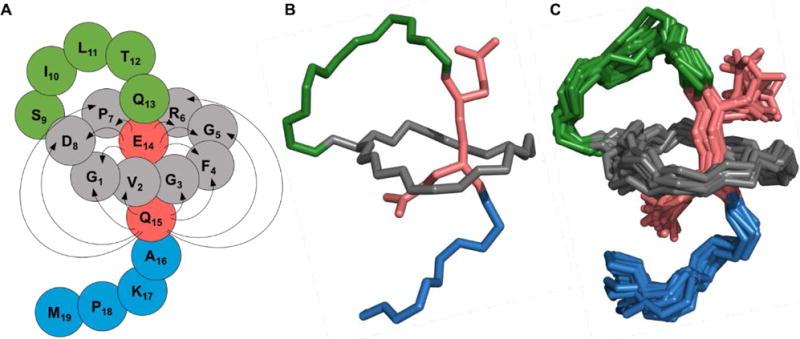
NMR structure of benenodin-1 ΔC5. A: Cartoon of the structure with grey residues representing the ring of the peptide, lime green residues in the loop, and blue residues in the tail. The steric lock residues Glu-14 and Gln-15 are shown in red. Arrows show NOEs observed between ring residues and steric lock residues. B: The lowest energy conformer of benenodin-1 showing the backbone of the peptide and the sidechains of the steric lock residues. Coloring is the same as in the cartoon in part A. C: As in part B, but showing the 20 lowest energy structures.
