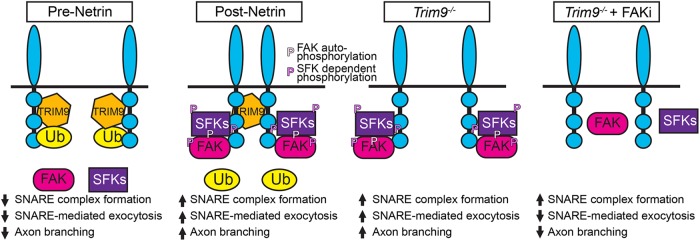
FIGURE 7:
Model of TRIM9-mediated regulation of signaling scaffolds. In the absence of netrin-1, DCC is ubiquitinated in the presence of TRIM9. The addition of ubiquitin inhibits FAK binding and downstream activation of FAK and SFK and constrains vesicle fusion and axon branching. The addition of netrin-1 results in loss of DCC ubiquitination and TRIM9-dependent clustering of DCC. FAK binds to the P3 domain of DCC and is autophosphorylated, leading to interactions with SFK and SFK-dependent phosphorylation events. This creates locally activated signaling complexes that promote vesicle fusion and axon branching. In the absence of TRIM9, the loss of DCC ubiquitination leads to aberrant interaction and activation of FAK and SFK, but a lack of signal complex clustering, leading to aberrant exocytosis and branching. Inhibition of FAK activity blocks subsequent SFK activity and SNARE-mediated exocytosis and branching.