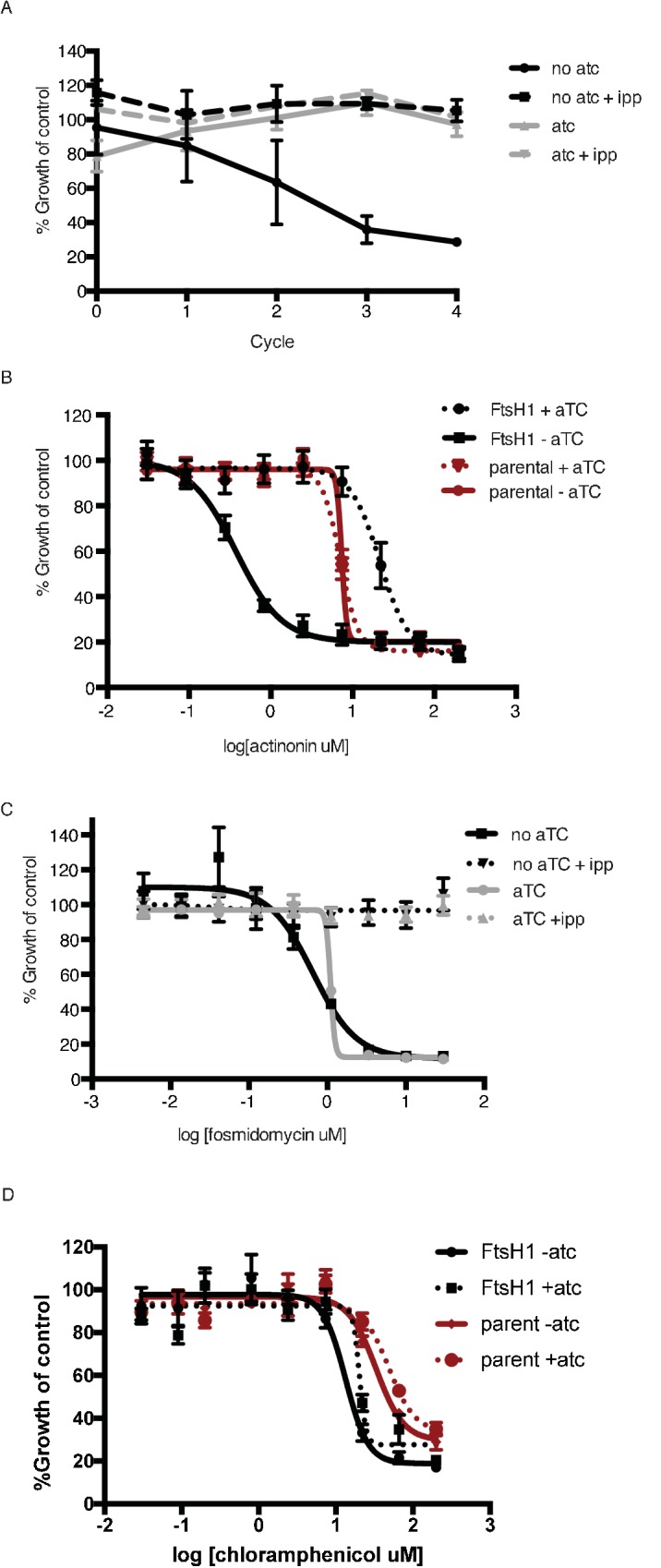
Figure 3. Knockdown of ftsH1 in P. falciparum leads to apicoplast loss and hypersensitivity to actinonin.
(a) Schematic of the endogenous knockdown strategy. When aTC is present in the media, the tet-repressor binds aTC and does not bind the 10x-aptamer sequence, which relieves translational repression, allowing PfFtsH1 to be expressed. When aTC is washed out of the media, the tet-repressor binds the 10x-aptamer and prevents expression of PfFtsH1. (b) Time course of parasite growth without aTC and in the presence or absence of IPP in the media, normalized to the untreated or IPP-rescued parental strain as appropriate. Error bars represent the SEM of two biological replicates. (c) Time course of the apicoplast:nuclear genome ratio measured by quantitative PCR (qPCR) using primers for the apicoplast and nuclear genomes during treatment with or without aTC. All samples contained IPP to rescue parasite growth. Genome ratios were normalized to respective parental cultures also grown with IPP. Error bars as in c. (d) Dose-dependent parasite growth inhibition by actinonin in the absence or presence of aTC. Error bars as in c.
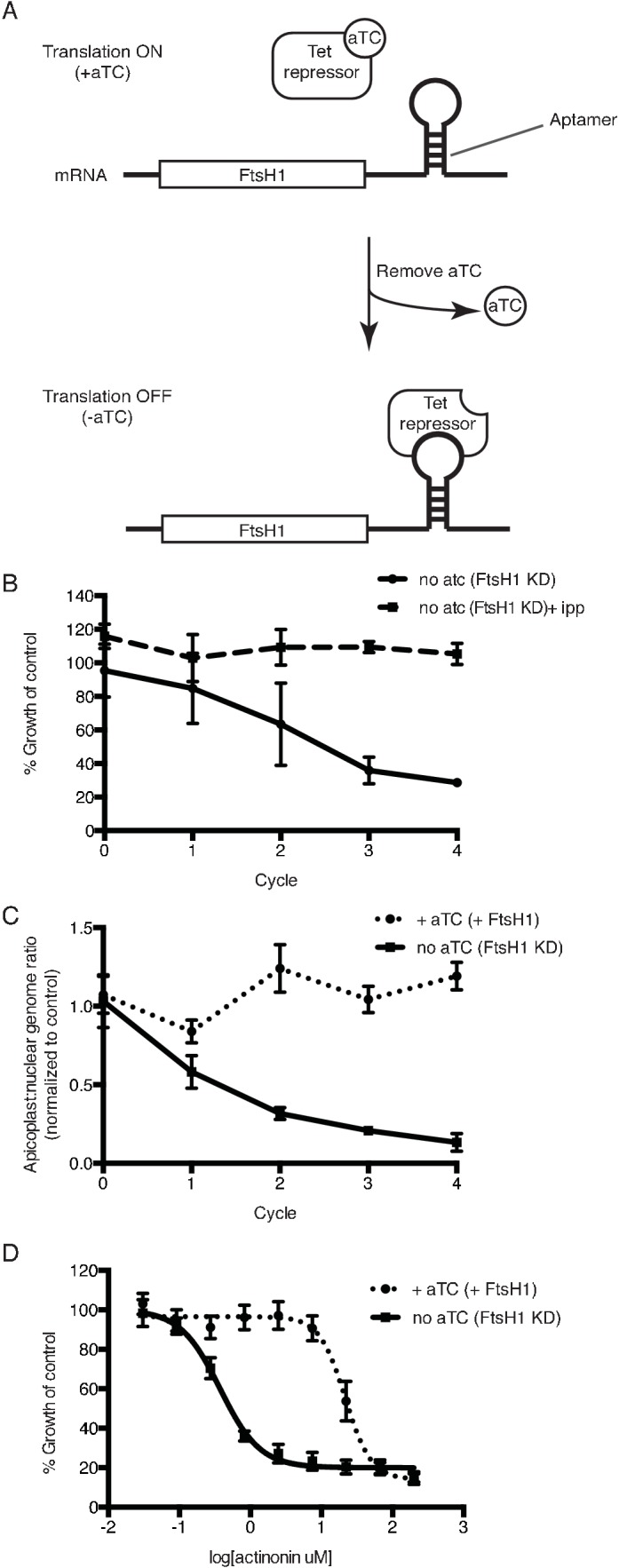
Figure 3—figure supplement 1. C-terminal cleavage of PfFtsH1 is dependent on the presence of the apicoplast and efficiency of PfFtsH1 knockdown can be assessed in parasites missing their apicoplast.
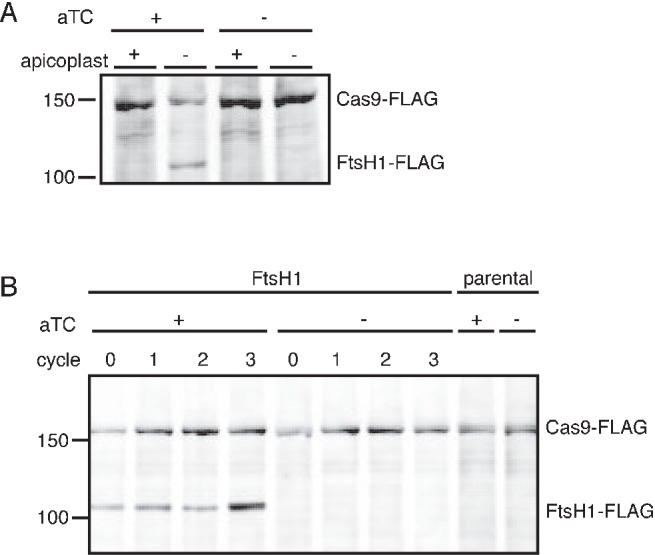
Figure 3—figure supplement 2. Knockdown of PfFtsH1 specifically disrupts the apicoplast and leads to specific hypersensitivity to actinonin.