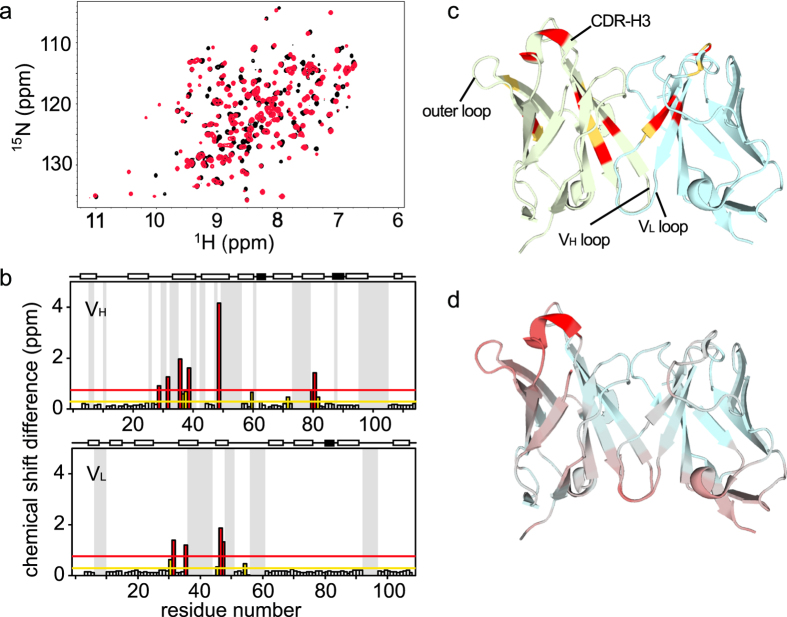
Figure 2.
The HyHEL-10 Fv fragment changes its structure upon binding to lysozyme. (a) HSQC spectra of free and bound HyHEL-10 are shown in black and red, respectively. (b) Chemical shift differences between the free and bound forms are plotted for the VH (top) and VL (bottom) domains. Above each panel, the α-helix and β-strand regions are shown as open and filled boxes, respectively. Yellow and red lines represent the average and average plus one standard deviation of the chemical shift differences, respectively. (c) The crystal structure of bound HyHEL-10 (PDBID: 1C08) with residues highlighted in the same colors as in (b). The VH and VL domains are shown in light green and light blue, respectively. (d) The RMSD between the crystal structure of the free (PDBID: 5AYU) and bound (PDBID: 1C08) forms of HyHEL-10, which are superimposed via the VL domain, is shown in a continuous color scheme from light blue to red, corresponding to RMSD values of 0.08 and 3.27, respectively. Crystal packing that possibly affects the RMSD values was observed in both the 5AYU and 1C08 structures particularly for residues in the crystal packing contact surface at the loops in both VL and VH, corresponding to the region colored in red.