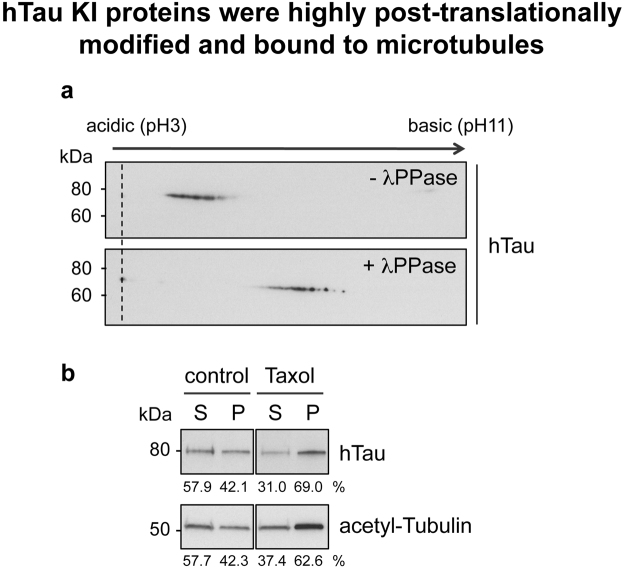
Figure 3.
hTau KI proteins were highly post-translationally modified and bound to microtubules. (a) 2D gel electrophoresis was performed on total protein extracts retrieved from hTau KI Drosophila heads (upper panel). hTau isovariants were detected using an anti-hTau antibody. Lambda phosphatase (λPPase) treatment of the protein extracts resulted in a drastic shift of hTau protein species towards basic pH (lower panel). (b) An in vivo microtubule-binding assay indicated that hTau proteins bound to microtubules in hTau KI Drosophila heads. Incubating protein samples with Taxol induced Tubulin polymerisation, as indicated by the increased amounts of acetyl-Tubulin retrieved in the pelleted fraction. hTau KI proteins precipitated with acetylated Tubulin in Taxol condition. The vehicle, i.e. DMSO, was used for control experiments. S: supernatant, P: pellet. Western blots were cropped in this figure; full blots are shown in Supplementary Figure 16.