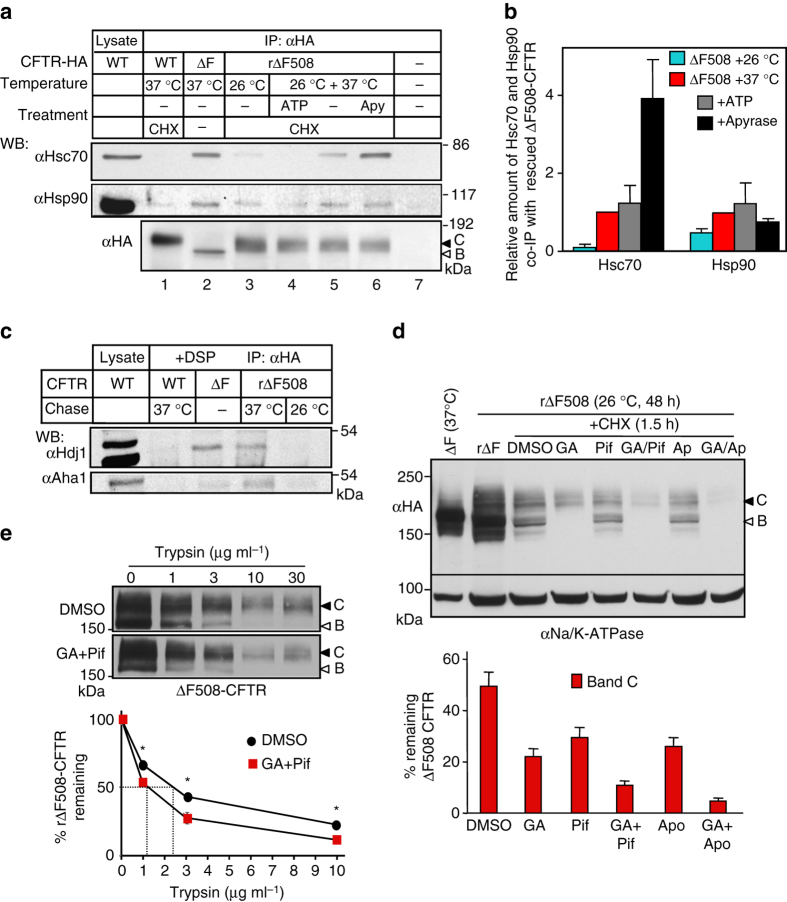
Fig. 1.
Molecular chaperone activity maintains the limited transport competence of ΔF508- and P67L-CFTR. a Hsc70 and Hsp90 association with WT- and ΔF508-CFTR containing a C-terminal HA-tag in BHK-21 cells by co-immunoprecipitation (Co-IP) and immunoblotting. Low-temperature rescued ΔF508-CFTR (rΔF508-CFTR, 24 h, 26 °C, lanes 3–6), the channel was either unfolded for 2.5 h at 37 °C in the presence of 150 μg ml−1 cycloheximide (CHX) (lanes 4–6) or exposed to 37 °C for 20 min and then cultured at 26 °C for 12 h with CHX. The latter protocol preserved the near-native conformation of the complex-glycosylated form (band C, filled arrowhead), while ensured degradation of the core-glycosylated form (band B, empty arrowhead, lane 3). WT-CFTR cells were exposed to CHX (2.5 h, 37 °C, lane 1). CFTR was IP with anti-HA antibody in the absence or presence of 2 mM ATP plus 1 mM MgCl2 ( + ATP) or with 150 U ml−1 Apyrase ( + Apy) and the IP was probed for Hsc70/Hsp90. Parental (lane 7) and non-rescued ΔF508-CFTR (lane 2) BHK-21 cells served as controls. b Hsc70 or Hsp90 fold-association with the complex-glycosylated rΔF508-CFTR was expressed relative to that observed after unfolding 37 °C for 2.5 h. Data are means ± standard error of the mean (SEM), n = 3–4. c Co-IP of co-chaperones with CFTR after crosslinking with 0.1 mM dithiobis[succinimidyl propionate] (DSP) was performed after exposing the cells to CHX chase (2.5 h, at 26 or 37 °C) as described for a. d The effect of Hsp70/Hsp90 inhibitors on the rΔF508-CFTR turnover, measured by quantitative immunoblotting. Rescued ΔF508-CFTR (26 °C, 48 h) was unfolded for 1.5 h at 37 °C in the absence or presence of Hsc70 (1 μM pifthrin μ [Pif] or 1 μM apoptozole [Apo]) or Hsp90 (10 μg ml−1 GA) inhibitor plus 150 μg ml−1 CHX. Na+/K+-ATPase served as loading control. Densitometric analysis of rΔF508-CFTR band C remaining after 1.5 h CHX chase. Data are means ± SEM, n = 3–4. e Limited trypsinolysis of ΔF508-CFTR in microsomes. Microsomes were isolated from BHK-21 cells that were exposed to DMSO or 5 μM Pif and 5 μg mlα GA (2 h, 37 °C) as described in Methods. Remaining complex-glycosylated rΔF508-CFTR was expressed as the percentage of the initial amount (bottom panel). Means ± SEM, n = 3–4