Fig. 3.
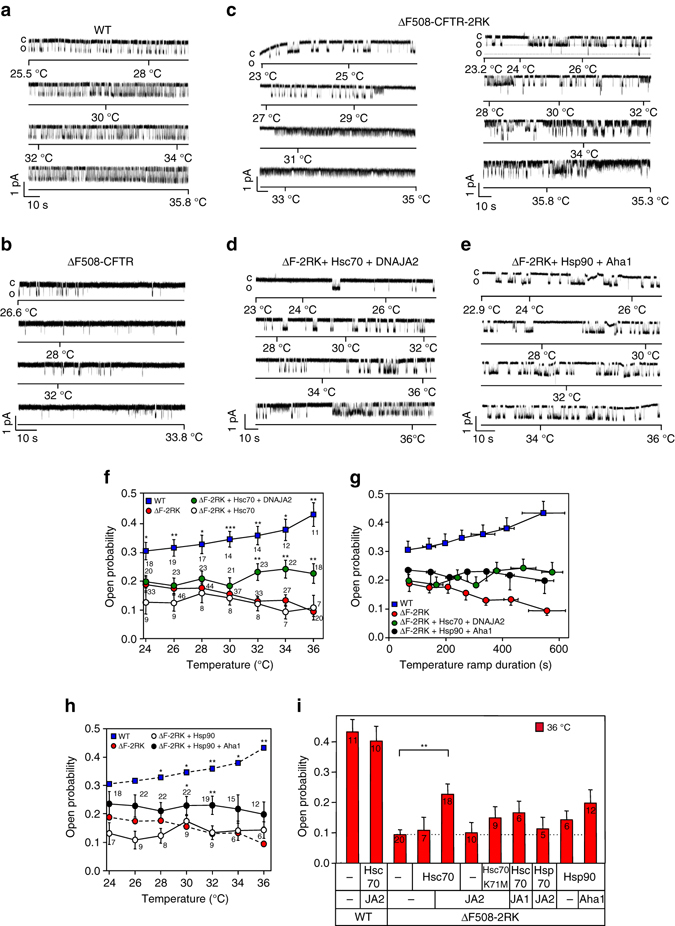
Molecular chaperones partially revert thermal inactivation of ΔF508-CFTR in black lipid membrane (BLM). a–e Single-channel records of WT-, ΔF508- and ΔF508-CFTR-2RK channel temperature-dependent activity in the absence a–c or presence of Hsc70 (2 µM) and DNAJA2 (2 µM) d or Hsp90 (2 µM) and Aha1 (2 µM) e. The predominantly observable thermal inactivation of ΔF508-CFTR-2RK is shown on the left of c. Phosphorylated channels were reconstituted and recorded in BLM as described in Methods. Closed (c) and open (o) states are indicated. Two channels were incorporated into the BLM in the right panel of c. f The effect of Hsc70 or Hsc70 + DNAJA2 on the temperature-dependent open probability (P o) of the ΔF508-CFTR-2RK determined in 2 °C intervals as in a–d. g Time-dependent changes in the P o of WT- and ΔF508-CFTR-2RK during temperature ramps in the presence of the indicated chaperones. h Hsp90/Aha1 (2 µM), but not the Hsp90 alone, confers resistance against thermal inactivation of ΔF508-CFTR-2RK. i Effect of molecular chaperones and co-chaperones on the mean P o of WT- and ΔF508-CFTR-2RK at 36 °C. The concentration of Hsp70, Hsc70-K71M and DNAJA1 (JA1) was 2 µM. Data are means ± SEM, *P < 0.05, **P < 0.01 or ***P < 0.001. P o values of ΔF508-CFTR-2RK in the absence of chaperones have been derived from data in Veit et al.34. Number of independent records for channel activity is indicated on f and h
