Fig. 7.
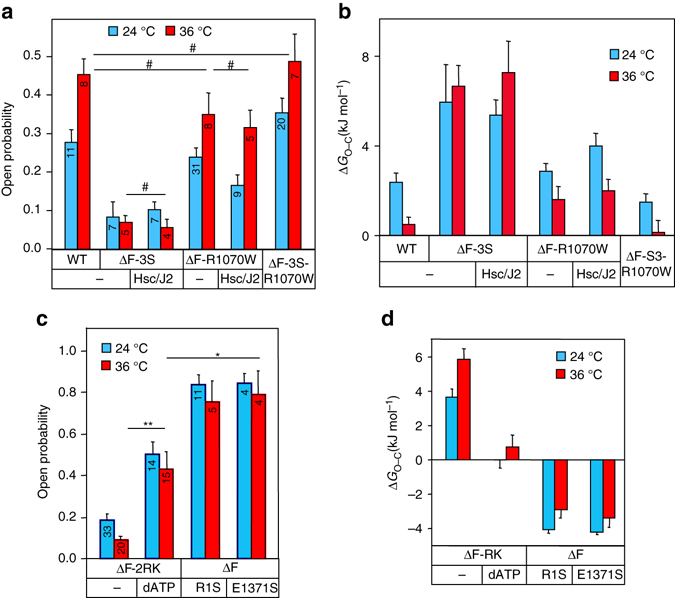
Chaperoning of ΔF508-CFTR unfolding is altered by second site mutations in the BLM. a, b The influence of second site suppressor mutations (3S, R1070W and 3S + R1070W) and Hsc70/DNAJA2 (Hsc/J2) activity on the P o a and ΔG O–C b of the ΔF508-CFTR-3HA at 24 and 36 °C in BLM. The P o and ΔG O were measured as in Figs. 3 and 4. c, d Stabilization of NBD1 and/or NBD1–NBD2 dimer was accomplished by R1S, E1371S mutations, or by the inclusion of 2ʹ-deoxyadenosine 5ʹ-triphosphate (dATP, 2 mM). The Gibbs free energy of opening was calculated based on the ΔG O–C = −RT(ln K e) equation. Representative records are shown in Supplementary Fig. 7a, b, d–f. The reconstituted channels were characterized as in Figs. 2 and 4. Data are means ± SEM, n is defined in c. # P ≥ 0.05, *P < 0.05 or **P < 0.01. The P o values of R1S and E1371S mutants were derived from Veit et al.34
