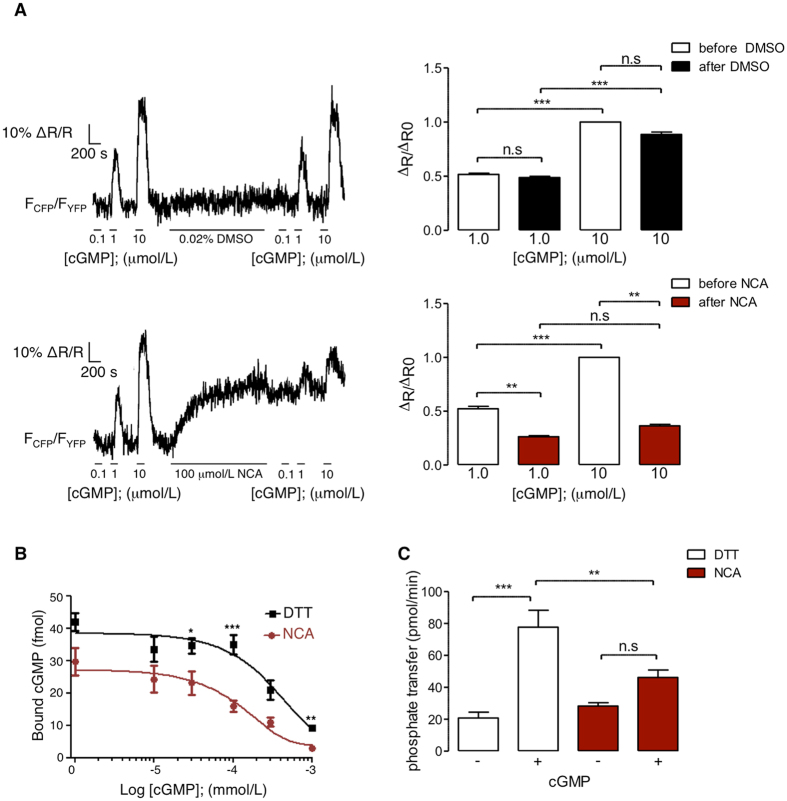
Figure 3.
Assessment of intradisulfide-induced changes in the cGMP-binding domain of PKGIα. (A) β-Escin-permeabilised primary mouse VSMCs stably expressing the FRET sensor cGi500 were superfused with intracellular-like medium (ICM) containing increasing concentrations of cGMP (0.1, 1, 10 µmol/L). This was followed by incubation with DMSO (0.02%; upper panel) or NCA (100 µmol/L; bottom panel) and by another incubation with ICM supplemented with increasing concentrations of cGMP (0.1, 1, 10 µmol/L). Changes of the FRET signals were recorded by epifluorescence microscopy. Representative FRET traces are shown on the left. The bar charts on the right summarise the FRET results from 10 cells per group as the ΔR/ΔR0 (amplitude relative to the signal induced by the first application of 10 µmol/L cGMP) induced by cGMP incubation before (white bars) or after exposure to DMSO (black; upper panel) or NCA (red; bottom panel). **P < 0.01, ***P < 0.001, comparing cGMP-induced changes in FRET ratio before and after DMSO or NCA by two-way ANOVA. (B) In vitro cGMP binding to PKGIα after exposure to DTT (100 mmol/L, 10 min) (black squares) or NCA (100 µmol/L, 30 min) (red dots) was investigated by measuring binding of (3H)cGMP in the presence of increasing concentrations of unlabeled cGMP (0, 10, 30, 100, 300, 1000 nmol/L). The data are representative of 5 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 comparing exposure to DTT or NCA at the same cGMP concentrations. (C) To assess PKGIα activity, in vitro kinase assays were performed using γ32P-ATP after DTT (100 mmol/L, 10 min) or NCA-treatment (100 µmol/L, 30 min), in the presence or absence of cGMP (300 nmol/L) with Glasstide as a substrate. Bar chart summarises data of 6 independent experiments. PKGIα activity was expressed as phosphotransfer into PKGIα substrate per minute. **P < 0.01, ***P < 0.001 intergroup comparison; n.s.: non-significant.