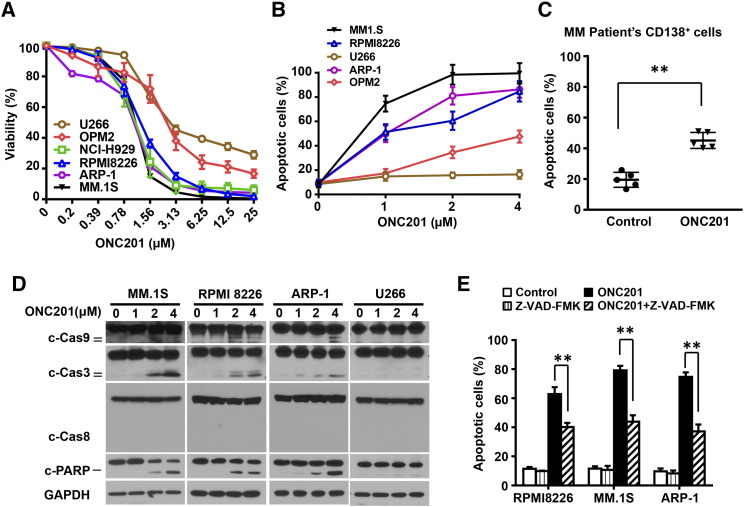
Figure 1.
Treatment of ONC201 reduces cell viability and induces apoptosis in both patient-derived myeloma cells and cell lines. Myeloma cells were cultured with gradually increased concentrations of ONC201 for 72 hours. After cultures, the cells were subjected to the Cell-Titer Glo Luminescent kit for assessing cell viability and the annexin-V binding assay for assessing cell apoptosis. (A) Shown is the percentage of cell viability in myeloma cell lines U266, OPM2, NCI-H929, RPMI8226, ARP-1, and MM.1S cells treated with different doses of ONC201. The IC50 of ONC201 treatment is 1 μM to 1.5 μM in the tested cells. (B-C) Annexin-V binding assay shows the percentage of apoptotic cells in (B) myeloma cell lines MM.1S, ARP-1, RPMI8226, OPM2, and U266 treated with 0 μM, 1 μM, 2 μM, or 4 μM of ONC201, and (C) CD138+ malignant plasma cells isolated from bone marrow aspirates of five patients, treated without (Control) or with 2 μM of ONC201. (D) Western blot analysis shows the cleaved (c) levels of caspases (Cas) 9, 3, and 8, and PARP in MM.1S, ARP-1, RPMI8226, and U266 cells treated with 0 μM, 1 μM, 2 μM, or 4 μM of ONC201. The levels of GAPDH served as protein loading controls. (E) Flow cytometry analysis shows the percentage of apoptotic cells in the myeloma cell lines MM.1S, ARP-1, and RPMI8226, treated with ONC201 without or with the pan-caspase inhibitor Z-VAD-FMK (50 μM). Results shown are representative of three to five independent experiments. **P < .01.