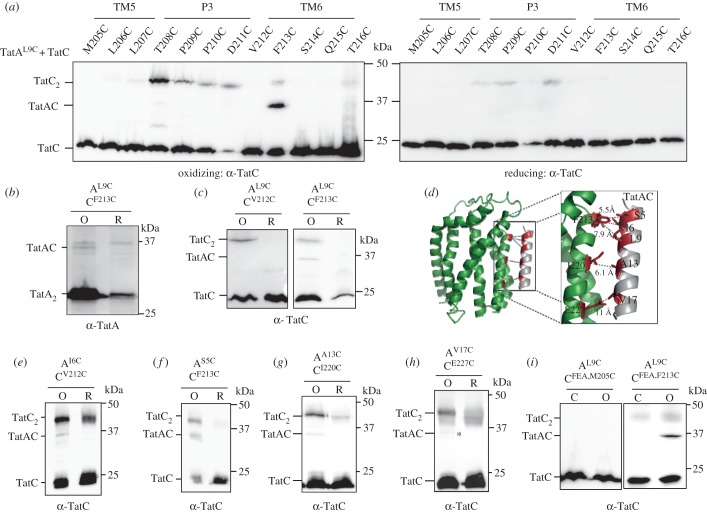
Figure 3.
TatAL9C interacts with TatCF213C in vivo. (a,e,g,h) Western blot analysis (separated on 12.5% polyacrylamide gels) of whole cells of E. coli strain DADE-P producing the indicated Cys substitutions in TatA and TatC (and wild-type TatB, from plasmid pUNITATCC4) following exposure to 1.8 mM CuP (oxidizing) or 10 mM DTT (reducing) for 1 min. Cross-linked products were visualized by immunoblotting using anti-TatC antibodies. The asterisk in (h) indicates a faint TatAC cross-link. (b) The TatAL9C–TatCF213C oxidized (O) and reduced (R) samples from (a) were separately probed with an anti-TatA antibody (note that the TatA monomer that is in large excess has been run off the bottom of the gel). (c,f) Cells of strain DADE harbouring plasmid pTAT101 producing (c) TatAL9C and wild-type TatB along with either TatCV212C or TatCF213C, or (f) TatAS5C, wild-type TatB and TatCF213C, as indicated, were incubated with 1.8 mM CuP (O) or 10 mM DTT (R) for 1 min. (d) Structural model of TatA interacting with TatC at the TatA constitutive binding site. The backbone distances between TatAS5/L9/TatCF213, TatAI6/TatCV212, TatAA13/TatCI220 and TatAV17/TatCE227 are shown. (i) Cells of strain DADE producing TatAL9C and wild-type TatB alongside TatCF94A,E103A,M205C or TatCF94A,E103A,F213C (annotated TatCFEA,M205C or TatCFEA,F213C, respectively) from pTAT101 were left untreated (C) or incubated with 1.8 mM CuP (O) for 1 min. For (c,d), following quenching, membranes were prepared, samples separated by SDS-PAGE (12.5% polyacrylamide) and immunoblotted using an anti-TatC antibody.