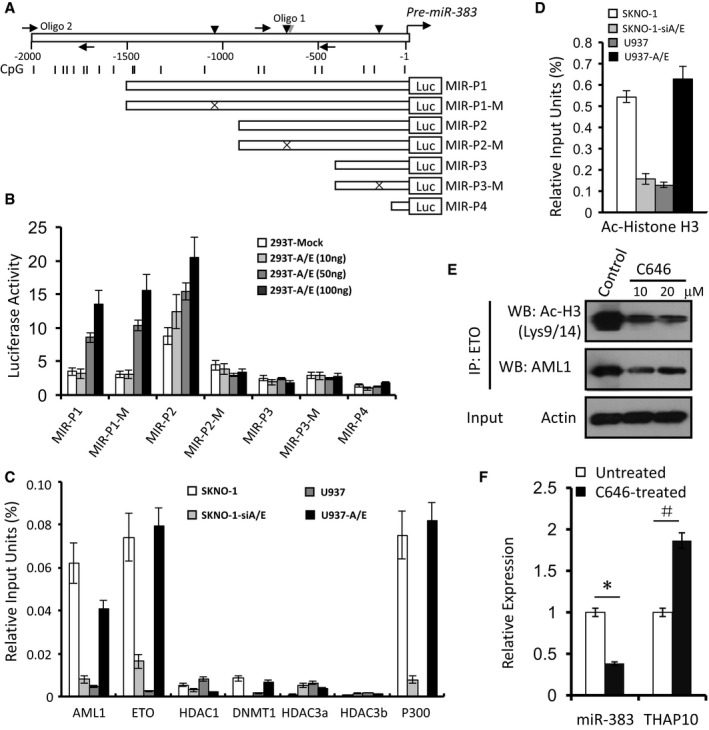
Upper panel: Schematic diagrams of the AML1‐ and p300‐binding sites and the CpG islands along the pre‐miR‐383 genes. Numbers indicate the nucleotides relative to pre‐miR‐383 (+1 nt). Vertical arrowheads indicate AML1 (dark) and p300 (grey) binding sites; horizontal arrows indicate the location of the primers used for the ChIP assays; vertical lines indicate CpG dinucleotides. Lower panels: A series of constructs containing different AML1‐binding sites and their mutants.
Luciferase reporter activities of human 293T cells transiently co‐transfected for 48 h with luciferase reporter constructs containing the wild‐type sequence of the miR‐383 regulatory regions or its mutant counterparts, together with increasing amounts of pcDNA3.0 containing AML1‐ETO or mock cDNA (293T‐Mock). Triplicate values are defined as mean ± SD.
ChIP using the indicated antibodies or IgG, after which qRT–PCR was performed to evaluate the specificity of protein binding. Triplicate values are defined as mean ± SD.
ChIP using Ac‐histone H3 antibody or IgG, after which qRT–PCR was performed for amplification of the region in miR‐383 containing the predicted AML1‐binding site in the upstream region of miR‐383 with the predicted p300‐binding site, to evaluate the specificity of protein binding. Triplicate values are defined as mean ± SD.
Kasumi‐1 cells were treated with C646 (10 or 20 μM) for 24 h, after which acetylation of histone H3 was analysed by immunoprecipitation (IP) and Western blot.
qRT–PCR quantification of miR‐383 and THAP10 mRNA levels in the Kasumi‐1 cells treated with or without C646 (10 μM) for 24 h. The results represent the mean of three independent evaluations ± SD (*P = 0.0001, #
P = 0.002). Student's t‐test was used for the comparisons.
Data information: All experiments were performed in triplicate.