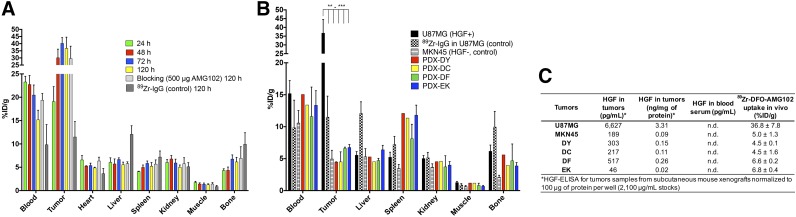
FIGURE 6.
(A) Biodistribution data from 89Zr-DFO-AMG102 in female nude mice bearing U87MG subcutaneous xenografts from 24 to 120 h after injection (∼0.74–1.11 MBq [∼20–30 μCi], ∼5 μg), including controls of 89Zr-IgG (nonspecific human IgG antibody) and blocking dose (500 μg, 100-fold cold AMG102 coinjected), showing substantial uptake in U87MG tumors with high local levels of HGF, low uptake of control 89Zr-DFO-IgG (EPR effect), and no apparent blocking effect (see “Discussion” section). (B) Biodistribution data at 120 h after injection showing 89Zr-DFO-AMG102 and 89Zr-DFO-IgG (control) (∼0.74–1.11 MBq [∼20–30 μCi], ∼5 μg) in U87MG (HGF+, MET+) and MKN45 (control, HGF−, MET+), and data from 4 different gastric PDXs at 120 h after injection showing low tracer uptake from 89Zr-DFO-IgG control in U87MG and 89Zr-DFO-AMG102 in MKN45, and similarly low uptake of 89Zr-DFO-AMG102 in the 4 PDX models (HGF levels previously unknown). (C) Data from ELISA assay for human HGF (pg/mL), and normalized HGF levels (ng/mg of protein) showing HGF levels in tumor homogenates and blood serum samples of tumor-bearing mice and corresponding in vivo %ID/g uptake values of 89Zr-DFO-AMG102. Statistical significance shown from Student unpaired t test using PRISM software, *P = ≤0.05, **P ≤ 0.01, ***P = ≤ 0.001, with all tumor uptake comparisons to U87MG having statistical significance between **P and ***P.