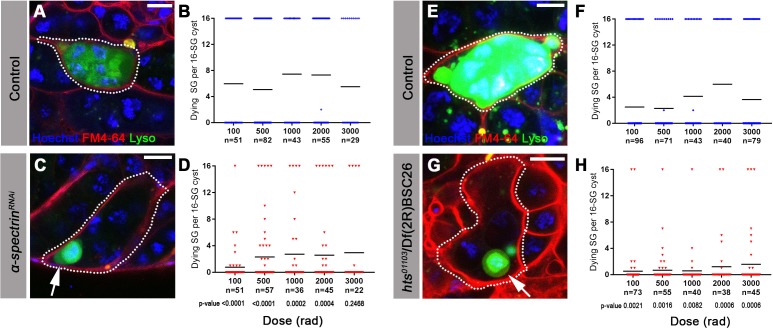
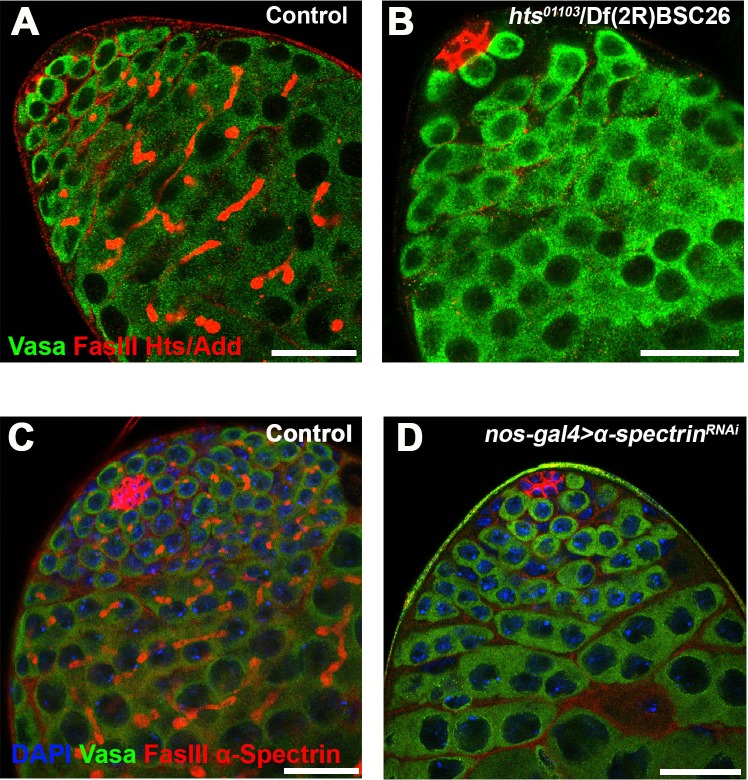
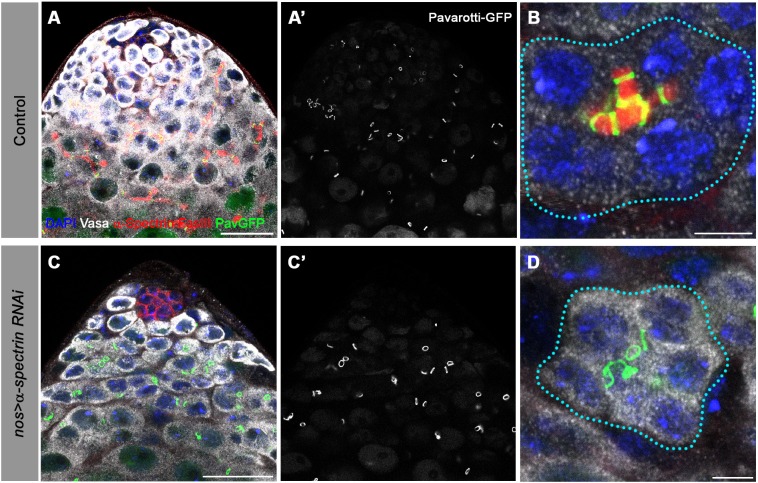
Figure 3. The fusome is required for synchronized all-or-none SG death within a cyst.
(A) A Lysotracker-positive 16-SG cyst (green, dotted outline) in unfixed control testes, with cyst borders marked by FM4–64 (red) and SG nuclei marked by Hoechst 33342 (blue). Bar: 7.5 µm. (B) Number of Lysotracker-positive cells within each 16-SG cyst of control testes at varying radiation doses. Black line, mean. n = number of 16-SG cysts scored. (C) A 16-SG cyst (dotted outline) in nos-gal4 >UAS-α-spectrinRNAi testes containing a single Lysotracker-positive SG (arrowhead). Bar: 10 μm. (D) Number of Lysotracker-positive cells within each 16-SG cyst of nos-gal4 >UAS-α-spectrinRNAi testes at varying radiation doses. Black line, mean. n = number of 16-SG cysts scored. P-values (comparing the corresponding radiation doses between control and mutant) determined by chi-squared test (See methods). (E, F) hts01103/+ control testes. Bar: 5 μm. (G, H) hts01103/Df(2R)BSC26 mutant testes. Bar: 7.5 μm.