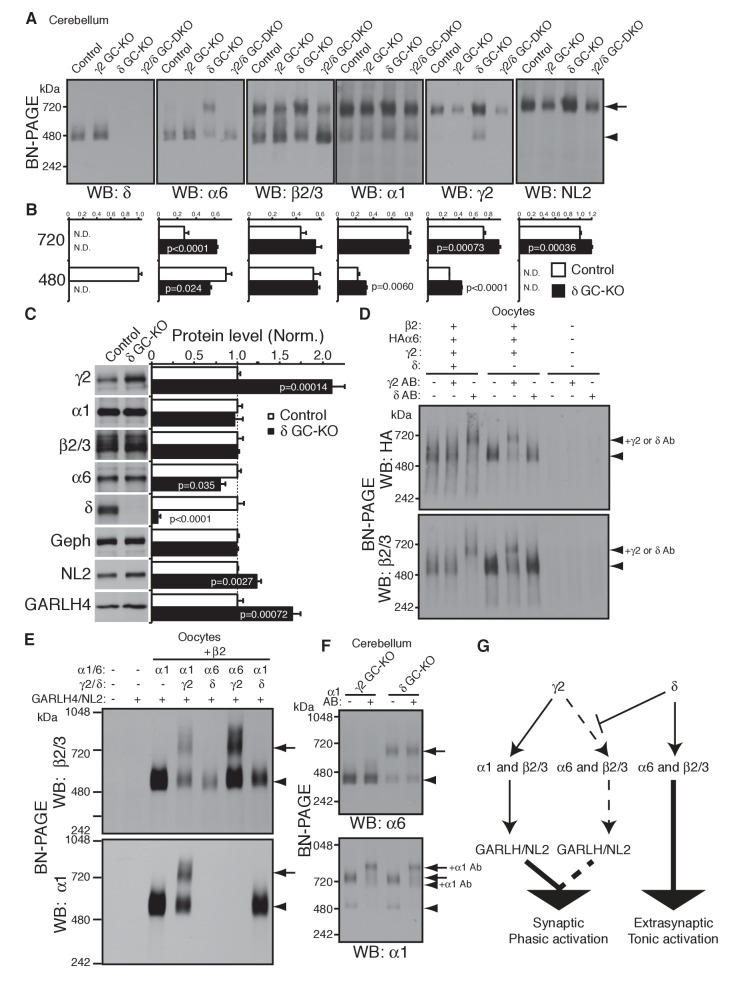
Figure 6. δ suppresses an assembly pathway for α6 with γ2, GARLH and NL2.
(A and B) δ suppresses incorporation of α6 into GABAAR/GARLH/NL2 complexes. (A) GABAAR complexes in cerebella from mice of various genotypes were examined by BN-PAGE. In control, γ2 GC-KO and γ2/δ GC-double KO cerebella, α6 expressed predominantly at 480 kDa. In contrast, in δ KO cerebellum, α6 expressed predominantly at 720 kDa, and an increase in signals of β2/3, γ2 and NL2, but not α1, at 720 kDa was also observed. As expected, in γ2 GC-KO cerebellum, γ2 signal was reduced but not eliminated. The residual γ2 signal originates from other cell types in the cerebellum that still expressed γ2. The images are representative of three independent experiments. (B) Relative ratios of the 720 and 500 kDa complex in cerebella from control and δ GC-KO (n = 5 animals). Signal intensity of each band was measured. Relative ratios of bands at 720 and 480 kDa were calculated in control mice, and relative changes in each band intensity in δ GC-KO were estimated. Data are given as mean ± s.e.m.; p values were determined with student's t test. (C) Total protein expression in cerebella from δ KO mice. Results are shown relative to control littermates (n = 5 animals each). Elimination of δ expression was confirmed and α6 was modestly reduced. A substantial increase in γ2, GARLH4 and NL2 was observed without changes in other GABAAR subunits (α1 and β2/3) or inhibitory synaptic marker protein Gephyrin (Geph). Data are given as mean ± s.e.m.; p values were determined with student's t test. (D) δ inhibits α6 assembly with γ2. GABAAR complexes from cRNA-injected oocytes were examined by BN-PAGE. In oocytes expressing HAα6, β2 and γ2 with or without δ, HAα6 and β2/3 migrated at 520 kDa. Addition of anti-δ antibody, but not addition of anti-γ2 antibody, to membranes from HAα6/β2/γ2/δ-expressing oocytes shifted HAα6 and β2 signal upward, indicating preferential assembly of α6 with δ relative to γ2. On the other hand, when δ was not present, addition of anti-γ2 antibody shifted HAα6 and β2 signal upward. The images are representative of four independent experiments. (E) Membranes from oocytes injected with the indicated cRNAs (0.2 ng ea for α1, β2, γ2 and GARLH4; 0.5 ng for δ; 1.0 ng for α6 and NL2) were analyzed using BN-PAGE. Upon co-expression with γ2, but not δ, both α1 and α6 assembled with β2 and formed complexes with GARLH4 and NL2 at 720 kDa. The images are representative of two independent experiments. (F) α1 and α6 segregate into distinct complexes, even when both associate with GARLH/NL2. GABAAR complexes in cerebella from γ2 GC-KO and δ GC-KO mice were examined by antibody shift assay on BN-PAGE. Addition of anti-α1 antibody shifted up α1 signal at 480 and 720 kDa in both genotypes. In contrast, in both genotypes, α6 signal was not shifted by addition of anti-α1 antibody. The images are representative of three independent experiments. The arrow and arrowhead indicate the GARLHed and GARLHless GABAAR, respectively, and antibody-bound complexes are indicated. (G) δ suppresses an assembly pathway for α6-containing GARLHed GABAARs. γ2 assembles with α1, β2/3 and GARLH/NL2 to mediate synaptic localization and phasic activation. Normally, δ sequesters α6, thereby suppressing γ2 interaction with α6. α6/δ-containing receptors do not interact with GARLH and neuroligin-2 (NL2), which are required for synaptic localization and phasic activation, and thus α6/δ-containing receptors localize at extrasynaptic sites and mediate tonic activation. In the absence of δ, α6 assembles with γ2, β2/3 and GARLH/NL2 to mediate synaptic localization and phasic activation.