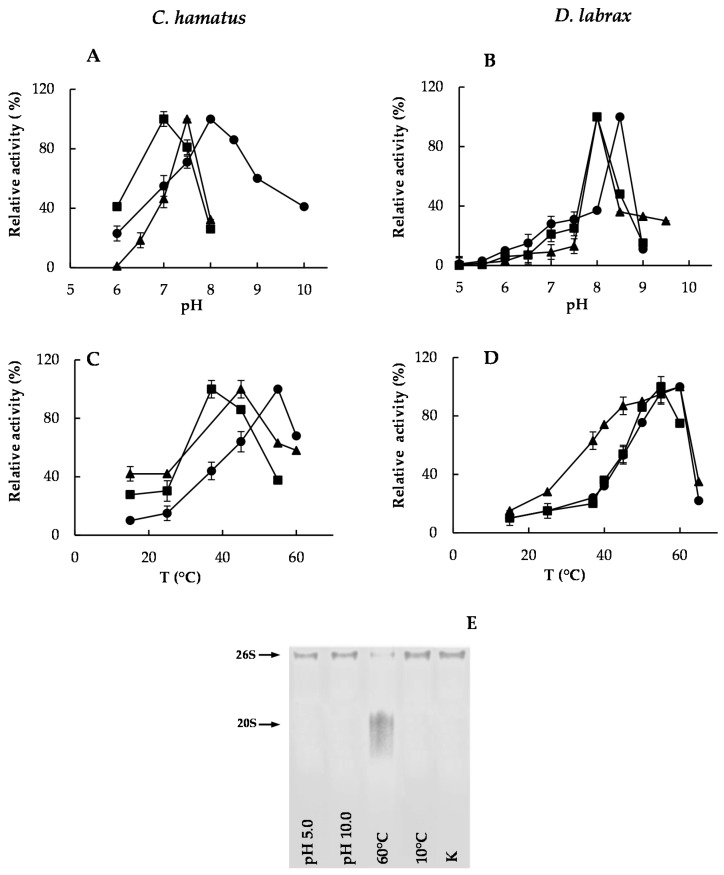
Figure 3.
Molecular properties of purified C. hamatus or D. labrax 26S proteasomes. (A,B) pH and (C,D) temperature effects on CT-like (circle), post-glutamate peptide hydrolase (PGPH)-like (square) and trypsin (T)-like (triangle) activities of 26S proteasomes. Relative activities are expressed as percentage of the corresponding maximal activities. All experiments were performed in triplicate on three different protein preparations. Three blank measurements (with no enzyme) at each pH and temperature value were performed. (E) Coomassie blue stained Native-PAGE of 26S proteasome from C. hamatus after pre-incubation for 5 min at extreme pH and temperature values. 26S proteasome pre-incubated for 5 min at pH 7.5 and 37 °C (K) was used as control. Data are expressed as means ± standard deviations. Standard deviation values lower than 5% are not shown.