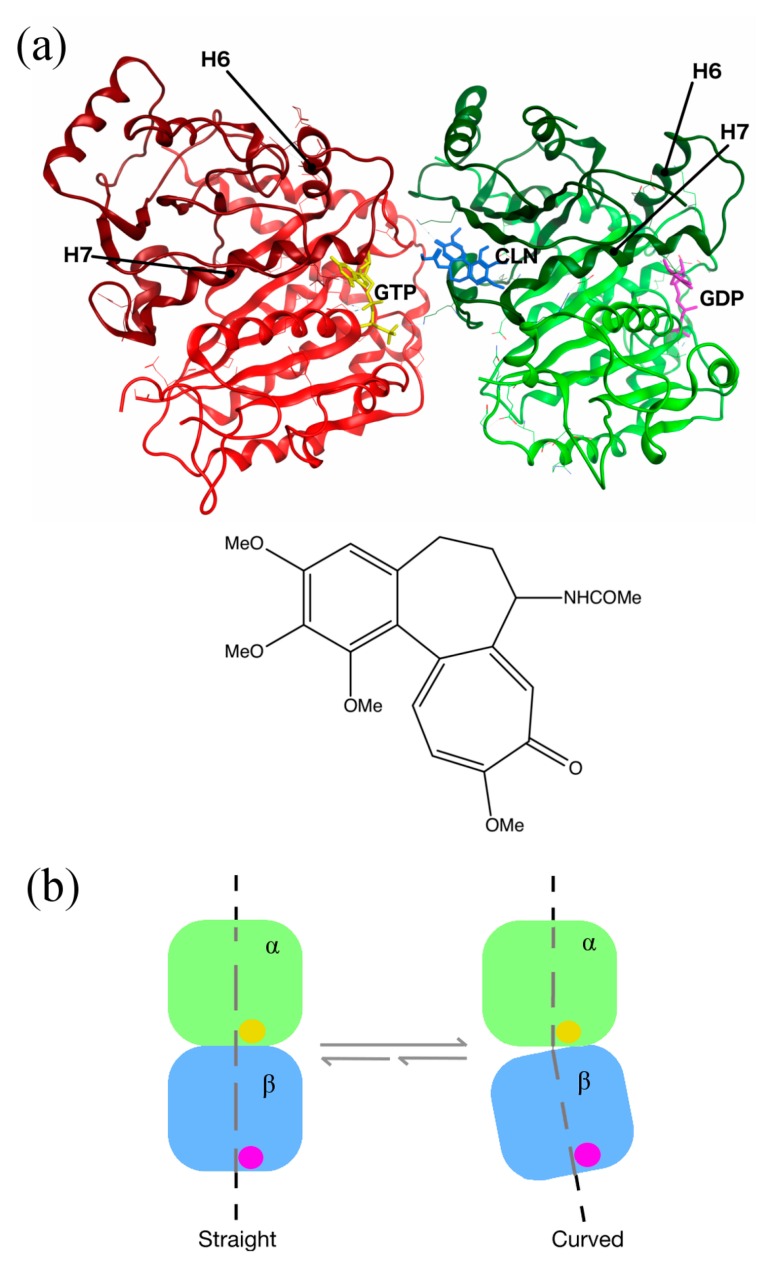
Figure 1.
Structure of human tubulin heterodimer bound to colchicine, guanosine triphosphate (GTP) and guanosine diphosphate (GDP). (a) Location of the colchicine-binding site: α and β tubulins are presented in red and green ribbon structures, respectively, with the intermediate domains in darker colors. GTP and GDP are shown in yellow and purple, respectively. Colchicine (CLN) is represented in blue, with a zoom on its chemical structure on the picture below (the image was prepared with MOE2012.10 [11], adapted from the Protein Data Bank (PDB) ID:1SA0). (b) Schematic representation of the conformational changes in tubulin, undergoing from straight to curved structures. The α subunit is bound to GTP (yellow ball), and the β subunit to GDP (purple ball). The tubulin dimer representation was redrawn based on the information obtained from Ravelli et al.’s 2004 study [6].