Table 1. Optimization of fluoroalkene synthesis a .
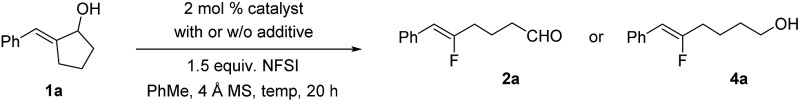
| ||||
| Entry | Catalyst | F+ (equiv.) | T (°C) | Yield b (%) |
| 1 | [Rh(cod)2]BF4 | NFSI (1.5) | 23 | 58 |
| 2 | [Ir(cod)Cl]2 | NFSI (1.5) | 23 | 60 |
| 3 | [RuCl2(p-cymeme)]2 | NFSI (1.5) | 23 | 59 |
| 4 | — | NFSI (1.5) | 23 | 61 |
| 5 | — | Selectfluor | 23 | <5 |
| 6 | K2CO3 | NFSI (1.5) | 23 | 55 |
| 7 | In the dark | NFSI (1.5) | 23 | 60 |
| 8 | Ag2CO3 | NFSI (1.5) | 23 | 54 |
| 9 | CuCl | NFSI (1.5) | 23 | 50 |
| 10 | — | NFSI (1.5) | 40 | 66 |
| 11 | — | NFSI (2.0) | 40 | 70 |
| 12 | — | NFSI (2.0) | 40 | 82 (4a) c |
aThe reactions were carried out with 0.2 mmol of 1a and 100 mg of a 4 Å molecular sieve in 2 mL of toluene open to air using commercially available NFSI.
bIsolated yields.
cThe isolated yield of alcohol 4a after the reduction of the aldehyde.
