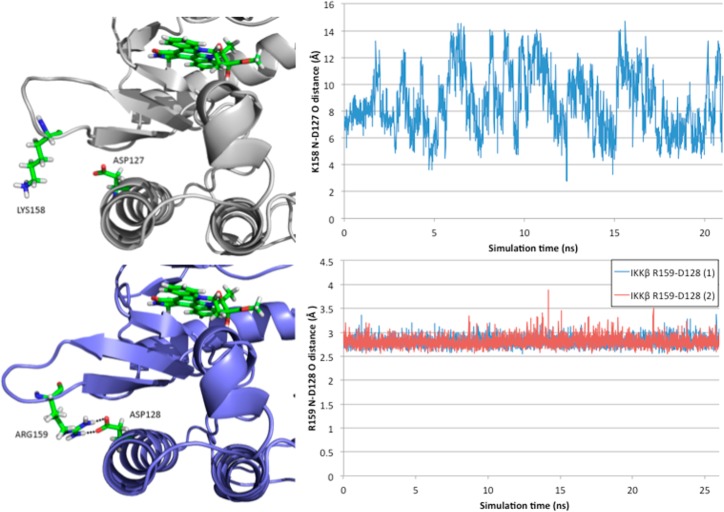
Figure 4.
(left) Loop conformation located below the active site in IKKα (white) and IKKβ (blue) and its relationship with α-helix 3 residue Asp127 (IKKα)/Asp128 (IKKβ). In IKKβ, Arg159 makes a reciprocal hydrogen bond dimer interaction with Asp128, whereas in IKKα Lys158 has no close interactions with Asp127. (right) Side chain amine nitrogen (Lys158 (IKKα)/Arg159 (IKKβ)) to side chain acid oxygen (Asp127 (IKKα)/Asp128 (IKKβ)) distance throughout the equilibrated phase of the simulation.