Figure 1.
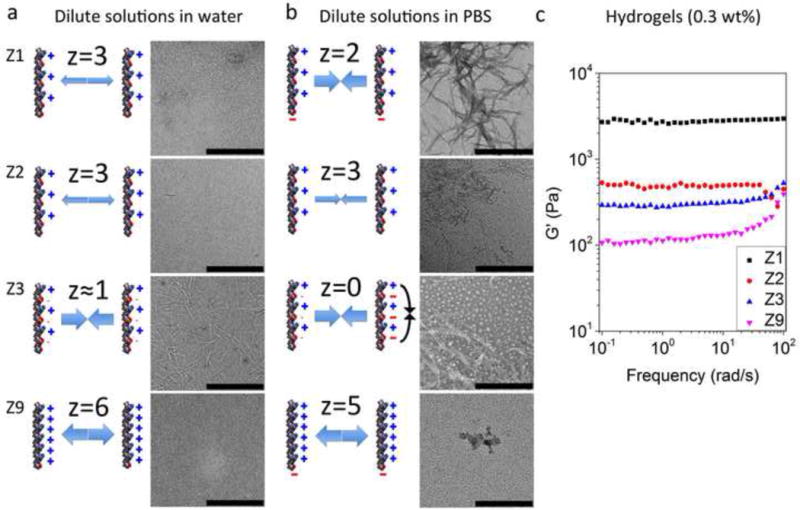
Charge distribution and density of peptide molecules significantly influenced their self-assembly behavior and the fiber forming ability. The net charges (z) of peptide molecules in water or PBS are indicated. (a) Dilute concentrations of peptides in water (pH 4.5) (Z1, Z2, and Z9) that have high charge density could not self-assemble into nanofibers due to the strong electrostatic repulsion, whereas the ionic self-complementary peptide Z3 with lower charge density formed well-defined nanofibers. (b) By increasing ionic strength with PBS (pH 7.4), the electrostatic repulsion of peptides with varied charge density was weakened due to charge shielding effect. Z1 with relatively lower charge density formed densely distributed nanofiber networks, whereas the fiber forming ability of Z2 and Z9 with higher charge density was partly or completely abrogated. A fraction of Z3 aggregated to form particles in PBS due to the intramolecular electrostatic attraction. The concentration of peptide dilute solutions is 100 μM. Scale bars represent 500 nm. (c) The stronger fiber forming ability of Z1 in dilute solutions formed much stronger hydrogels at higher concentration in PBS.
