Figure 1.
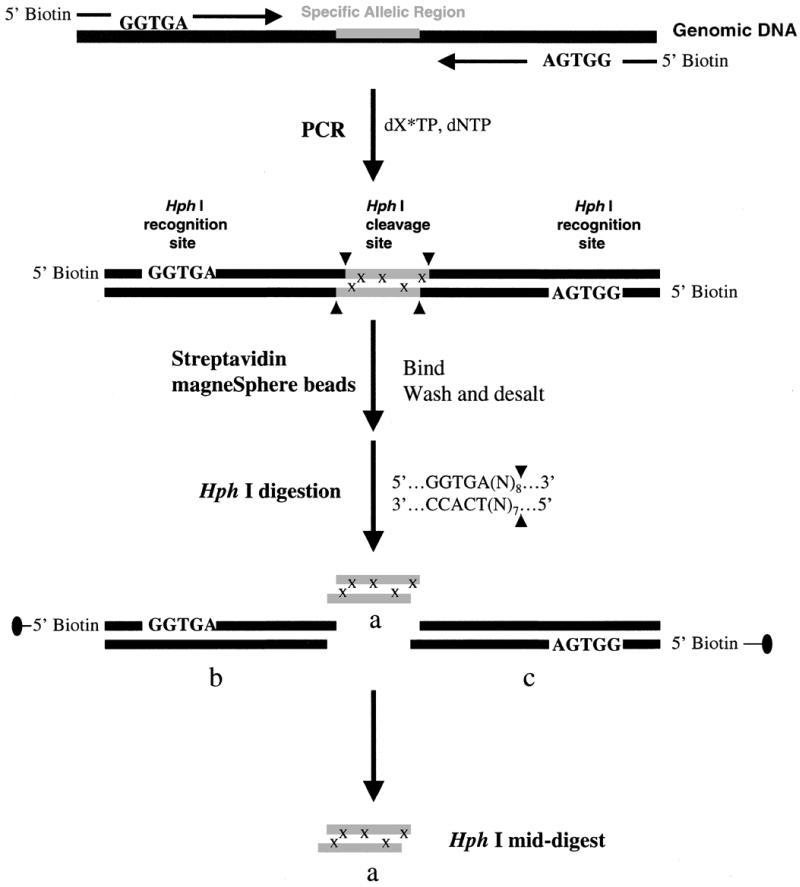
A schematic representation of our strategy for efficient and high throughput SNP detection. The synthetic PCR primers are biotinylated and the restriction recognition site of HphI is included at the 5′-end of each primer. HphI has the recognition sequence of 5′…GGTGA (N8)…3′, which dictates the restriction cleavage at the 8th nucleotide from the 5′ end or 7th from the 3′ end. The nucleotides, N, representing the complimentary sequences of the genomic DNA are included in the primer sequences. After PCR amplification and labeling with 13C/15N-labeled nucleotides, x, streptavidin magneSphere paramagnetic beads are added to the solution to capture the biotinylated PCR products through biotin–streptavidin interactions. The PCR products linked to the beads are washed and then subjected to the HphI restriction digestion, and the target sequence containing SNP site(s), HphI mid-digest, or a, is released in solution. The biotin-labeled end of the digest portions [HphI recognition sequence plus (N)8/(N)7 flanking regions], b and c, are attached to the paramagnetic beads and trapped with a magnesphere technology magnetic separation stand. The labeled HphI mid-digest, a, is then desalted via a nitrocellulose membrane and dried for MALDI-TOF analysis. In MALDI-TOF spectra, the 13C/15N-labeled nucleotides, x, will induce a mass shift in product a, away from the molecular mass of the unlabeled product. This mass shift gives the number of labeled nucleotides incorporated in the amplified product.
