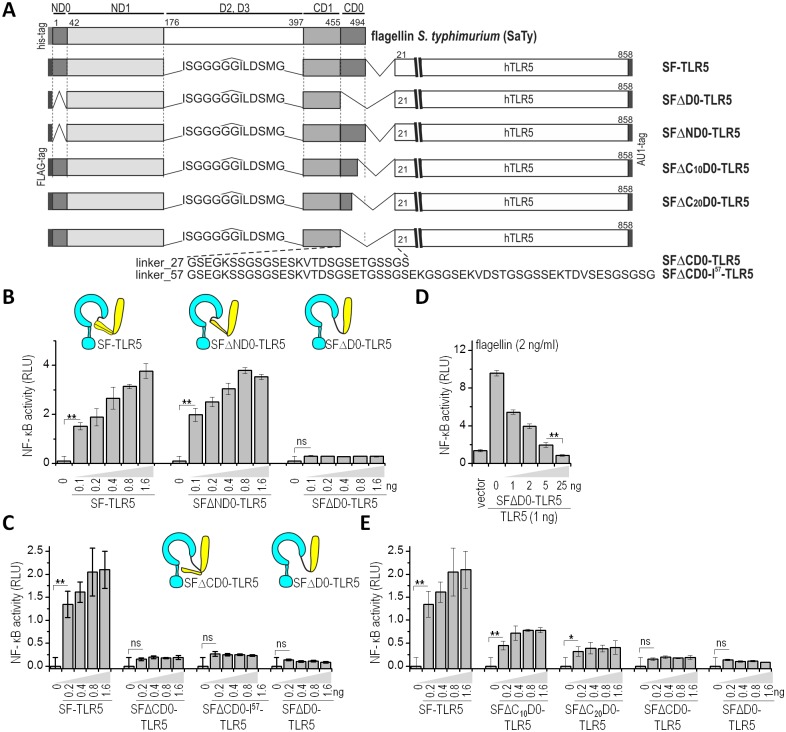
Fig 1. The C terminal D0 domain of flagellin is required for TLR5 activation.
(A) A schematic representation of the domain organization of S. typhimurium flagellin (SaTy) and of the chimeric constructs comprised of truncated flagellins linked to TLR5. Numbering refers to SaTy (UniProtKB - P06179) and TLR (UniProtKB: O60602). (B) Deletion of the N-terminal D0 domain (ND0) does not affect NF-κB activity. HEK293 cells were transfected with increasing amounts (ng) of plasmids encoding ligand-receptor fusions: SF-TLR5 as a positive control, SFΔND0-TLR5, or SFΔD0-TLR5. (C) Deletion of the C-terminal D0 domain (CD0) abrogates activity of the constitutively active fusion protein SF-TLR5. HEK293 cells were transfected with increasing amounts (ng) of plasmids expressing ligand-receptor fusions: SF-TLR5, SFΔCD0-TLR5, SFΔCD0-l57-TLR5, or SFΔD0-TLR5 (D) The D0 deletion construct SFΔD0-TLR5 forms inactive heterodimers with TLR5. HEK293 cells were transfected with TLR5 (1 ng) and increasing amounts of SFΔD0-TLR5 (1–25 ng). (E) Deletion of the C-terminal 10 (SFΔC10D0-TLR5) or 20 (SFΔC20D0-TLR5) amino acid residues of the C-terminal D0 domain decreases activity of the constitutively active fusion protein SF-TLR5. HEK293 cells were transfected with plasmids encoding SF-TLR5, SFΔC10D0-TLR5, SFΔC20D0-TLR5, SFΔCD0-TLR5, or SFΔD0-TLR5. (B-E) HEK293 cells were transfected with plasmids encoding the ligand-receptor fusions and NF-κB-dependent firefly and constitutively expressed Renilla luciferase activities were measured 18 h post-transfection. (Data are representative of three independent experiments. Bars represent the means of four biological replicates ±s.d.; **p<0.005; *p<0.05; nsp>0.05) See also S1 Fig.