Abstract
OBJECTIVES
To examine the relationship between a measure of subepidermal moisture (SEM) and visual skin assessment (VSA) of erythema and Stage 1 pressure ulcers (PUs) performed a week later in nursing home (NH) residents.
DESIGN
Descriptive, cohort study.
SETTING
Two NHs.
PARTICIPANTS
Thirty-five residents.
METHODS
Concurrent VSAs and SEM readings were obtained at the sacrum, right and left trochanters, buttocks, and ischial tuberosities weekly for 52 weeks. SEM was measured using a handheld dermal phase meter, with higher readings indicating greater SEM (range 0–999 dermal phase units [DPUs]). VSA was rated as normal, erythema/Stage 1 PU, or Stage 2+PU. SEM was modeled as a predictor of VSA of erythema and PUs 1 week later (controlling for clustering), with concurrent moisture, Braden Scale PU risk status, anatomic site, and ethnicity as covariates.
RESULTS
Participants had a mean age of 84.7, 83% were female, and 80% were non-Hispanic white. SEM measures were lowest for normal skin (97 ± 122 DPU), higher for erythema/Stage 1 PUs (192 ± 188 DPU), and highest for Stage 21PUs (569 ± 320 DPU) across all sites (all P <.001). SEM was responsive to changes in VSA, and higher SEM predicted greater likelihood of erythema/Stage 1 PU the next week (odds ratio = 1.26 for every 100-DPU increase in SEM, P = .04).
CONCLUSION
SEM measures are associated with concurrent erythema and PUs and future (1 week later) development of erythema/Stage 1 PUs. SEM may assist in predicting early PU damage, allowing for earlier intervention to prevent skin damage.
Keywords: erythema, Stage 1 pressure ulcers, moisture
Pressure ulcers (PUs) are a common occurrence in nursing home (NH) residents, the majority of which are Stage 1 and 2 ulcers, with annual incidence rates of 2.2% to 24% reported.1,2 Stage 1 PUs account for up to 47% of all PUs in older people,3,4 and it has been suggested that they are predictors of more-severe PU development. Recently, in a study of 68 acute care patients and 115 long-term care residents with erythema or a Stage 1 PU identified, 14% deteriorated to more-severe ulcers (22% in acute care over 7 days and 9% in long-term care over 14 days).5 Other studies have shown that Stage 1 PUs deteriorate to more-severe ulcers, with proportions ranging from 26% (10/38) to 58% (11/19) in hospitalized and long-term care patients, respectively.6,7 Moreover, erythema has been shown to be a strong predictor of Stage 1 and 2 PUs, is stable over time, and occurs frequently in incontinent NH residents.8–10 Thus, detection of erythema and Stage 1 PUs is significant, because early intervention in these early-stage PUs may prevent deterioration to more-severe ulcers.
Early-stage PUs have also been suggested as an outcome measure of PU prevention care processes in NHs because they occur more frequently than Stage 3 and 4 PUs.1,8 However, detection and accurate identification of erythema and Stage 1 PUs with the standard method, visual skin assessment (VSA), is unreliable and often fails to detect skin color changes in darkly pigmented skin. In a recent prevalence study of acute care patients, Stage 1 PUs accounted for 48% of all ulcers in Caucasian patients but only 20% of all ulcers in African-American patients.4 This difference in reported prevalence likely reflects difficulties in detecting early-stage PUs in darker skin.
Historically, a Stage 1 PU was defined as persistent redness or nonblanchable erythema of intact skin, and detection was accomplished using VSA.11 In 1998, the National Pressure Ulcer Advisory Panel revised the definition of a Stage 1 PU to include nonvisual characteristics independent of skin color to better encompass persons with darkly pigmented skin. The additional characteristics include skin temperature, skin consistency (hardness or boggy feel), and sensation (pain or itching).12
Despite the more comprehensive definition of Stage 1 PUs, researchers and clinicians continue to depend on VSA for detecting early PUs. The use of the original definition of Stage 1 PUs remains evident in studies published since 1998, and there have been minimal attempts to validate the revised definition.13–16 Noninvasive biophysical measures of skin integrity that do not rely on skin color to detect erythema and Stage 1 PUs are few and have not been well studied.
Inflammation is the earliest sign of impending ulceration in PUs and diabetic foot ulcers.17,18 Inflammatory changes with resultant tissue edema can occur from 3 to 10 days before there is skin breakdown.18,19 In an example of a biophysical measure used to identify inflammation, one study found that persons with diabetes mellitus could evaluate surface skin temperature using a handheld infrared skin temperature probe to monitor for signs of impending ulceration.20 In a randomized, controlled trial of 85 patients with diabetes mellitus assigned to standard therapy or enhanced therapy using an infrared thermometer, the standard therapy group was 10.3 times as likely to develop a foot complication as patients using the handheld infrared thermometer to assess skin temperature and, if elevated, see a healthcare practitioner (95% confidence interval = 1.2–85.3). As another example, dermatologists have used tissue reflectance spectroscopy as a common noninvasive measurement of skin color changes before and after skin treatments in office or clinic settings.21
Subepidermal moisture (SEM) can be used to assess the epidermal barrier function of skin. SEM has been associated with maintaining a mature epidermis and is related to initial epidermal wounds.22,23 Surface electrical capacitance can be used to measure SEM and to evaluate reestablishment of an intact stratum corneum (the outermost layer of the skin) after injury with formation of the epidermal barrier as a function of hydration. The impedance of the skin to electrical forces is used to calculate surface electrical capacitance, which directly reflects the moisture in the epidermal tissues. Surface electrical capacitance has been used to quantify wound healing in burn patients and to examine the relationship between SEM and chronic wound healing in small case series.22,24–26 One study measured SEM at PU wound margins to assess periwound edema at four standardized sites in a small case study of six patients with spinal cord injury and chronic nonhealing Stage 3 or4PUs. The authors found higher SEM values at wound margins than at control sites. They suggested that periwound edema can be detected using SEM and that, as tissue edema decreases, the potential for healing increases.26 Nevertheless, no data exist comparing SEM measures with visual detection of erythema and Stage 1 PUs in NH residents.
The purpose of this study was to examine the relationship between SEM and direct VSA of erythema and Stage 1 PUs. The following research questions are addressed in this article: Does a relationship exist between SEM and visual detection of the presence of erythema/Stage 1 PUs? Can SEM measures be used to predict the presence of or changes in erythema and Stage 1 PUs?
METHODS
Subjects and Setting
This study was conducted as part of a large randomized trial to improve nutrition in NH residents. Residents were recruited from one nonprofit and one proprietary NH in the Los Angeles area. Residents were eligible for the skin health study if they were participating in the larger nutrition trial. The University of California at Los Angeles Human Subject Protection Committee approved the protocol. Research staff obtained written informed consent to participate in the current study from residents who were able to provide informed consent or from their designated representatives (in residents unable to provide consent) with assent obtained from the resident. Figure 1 shows the flow of participants for this study.
Figure 1.
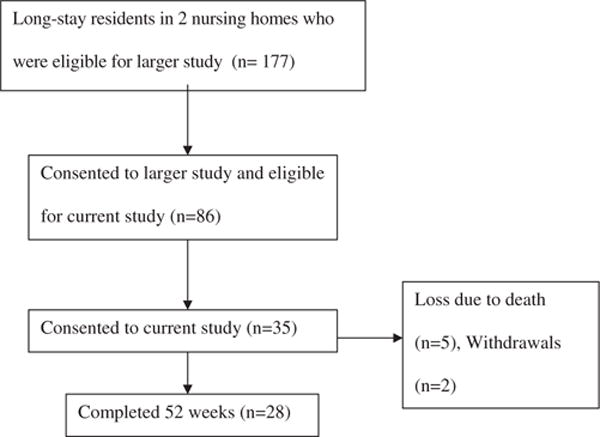
Flow of participants through the study. Of the 35 (40% consent rate) consented subjects, 28 completed the 52 weeks of the study, two withdrew, and five died.
Medical Record Data
At baseline, research staff extracted medical and demographic information from all consented participants’ charts and their most recent Minimum Data Set (MDS) assessment. Medical records were reviewed monthly to identify changes in care.
Braden Scale Risk Assessment Scores
Research staff assessed participants’ risk for PU development each month using the Braden Scale. The Braden Scale comprises six subscales that conceptually reflect degrees of sensory perception, moisture, activity, mobility, nutrition, and friction and shear.27,28 All subscales are rated from 1 to 4 (4 = best, 1 = worst for subscale), except for friction and shear, which is rated from 1 to 3. The subscales may be summed for a total score, with a range from 6 to 23, with lower scores indicating lower function and higher risk for developing a PU. In older patients, cutoff scores of 17 or 18 have been shown to be predictors of risk status.28 In the current study, interrater agreements tested by comparing two raters’ total scores on 31 cases of observations resulted in a Pearson correlation of 0.90 (P < .001). Interrater agreements on being at risk (total score≤18) versus not being at risk (total score 19–23) in these 31 cases resulted in a kappa statistic of 0.69 (P <.001).
Skin Assessment
Trained registered nurses assessed skin health through direct, independent VSAs each week. Before the project, extensive training was provided in detecting skin conditions, particularly for Stage 1 PUs, because this condition may be transient and difficult to detect.5,12 PUs more severe than Stage 1 were staged using the National Pressure Ulcer Advisory Panel’s four-stage definition.29 Stage 1 PUs were determined by evaluation of erythema, blanching, and tissue resilience. Erythema was graded as minimal, moderate, or severe discoloration, with minimal defined as pinkness or slight redness of the skin, moderate as bright redness in lightly pigmented skin and purple in darkly pigmented skin, and severe as dark red to purple in lightly pigmented skin and black to blue-grey in darkly pigmented skin. Blanching was defined as blanchable versus nonblanchable based on palpation. Resilience was determined by palpation and defined as normal elasticity of the tissue; bogginess or decreased stiffness; and indurated, hard, or increased stiffness. Stage 1 PUs were defined as moderate or severe skin discoloration with nonblanching and any level of tissue resilience. Erythema was defined as moderate or severe skin discoloration with blanching or with tissue resilience that was boggy or indurated. For analysis, erythema and Stage 1 PU categories were combined. VSAs covered seven anatomic locations: the sacrum and the right and left trochanters, buttocks, and ischial tuberosities. The presence or absence of urine or fecal incontinence was also recorded.
Interrater agreement was tested by comparing two raters’ findings on 38 cases of observations for the identification of the presence of erythema, erythema severity, and PU stage. Interrater agreement for presence of erythema at the sacrum, right and left ischium, and buttocks resulted in kappas ranging from 0.73 to 1.00, and for severity of erythema (blanchable vs nonblanchable), kappas ranged from 0.74 to 0.93 for the same sites. Interrater agreement on PU stage was 1.00 on 10 PUs in the 38 cases of observations.
SEM Measures
Concurrent with the VSAs, SEM was measured weekly at each of the anatomic locations using the NOVA Petite dermal phase meter (NOVA Technology Corporation, 75 Congress St., Portsmouth, NH). This is a standard technique for measuring skin hydration and is used commonly in the medical and cosmetic industry.30,31 A small probe was used to take readings at each of the seven anatomic locations. Readings were taken by placing the probe on the skin surface for 5 seconds, after which the impedance value of the skin was displayed in dermal phase units (DPUs). The DPU is an arbitrary relative value, and readings range from 0 to 999, with higher readings indicating higher SEM. SEM readings were taken immediately after a subject’s diaper or clothing was removed and before the VSAs were completed. Observers conducted the SEM measures and the VSAs but were blinded to the purpose of using the dermal phase meter. Three readings with the dermal phase meter were taken at each anatomic site. The three readings were highly correlated (correlation coefficient (r) = 0.90); thus, data from the third reading were used in the analysis. The third reading was used because of simultaneous skin surface temperature data collection, which requires longer probe placement for accuracy and is not relevant to this report. Interrater reliability was r = 0.63 for all sites. When urinary or fecal incontinence was present, the measure was taken after incontinence care was provided (e.g., the skin cleansed and patted dry). The impedance measure is independent of ambient temperature and humidity in the room, making it ideal for use in a NH environment.
Statistical Analysis
To assess the relationship between VSA and SEM, techniques were used that allowed for repeated measures, because an observation was defined according to week and anatomic location. The number of weeks when VSAs and SEM measures could be collected varied according to resident, with 28 residents having 44 or more weeks of data, two having 20 to 30 weeks of data, two having 10 to 15 weeks of data, and two having less than 10 weeks of data. Of the participants with long observation periods, only one had no visible skin damage at any site for the duration of the study. In all analyses, the VSA data for erythema (e.g., moderate or severe discoloration, blanchable, or with tissue resilience boggy or indurated) was combined with Stage 1 PU (e.g., moderate or severe, nonblanchable, and any level tissue resilience) data. Thus, erythema/Stage 1 data include all visual assessments of moderate or severe discoloration that were blanchable or nonblanchable with any level of tissue resilience.
Separate repeated measures analyses of variance (ANOVAs) for each anatomic location were used to determine whether mean moisture value varied according to VSA, followed by pairwise post hoc comparisons. A repeated-measures ANOVA with pairwise post hoc comparisons was also used to determine whether SEM measures were responsive to weekly changes in visual skin condition. Change scores for SEM measures for each week were calculated, and visual skin condition changes were rated in one of three patterns of skin change. The skin change patterns were rated as no change, any level of skin damage to the same level of skin damage (e.g., no damage to no damage); deterioration, any level of skin damage to more severe skin damage (e.g., no damage to erythema/Stage 1 PU); and improvement, any level of skin damage to less-severe skin damage (e.g., erythema/Stage 1 PU to no damage).
To determine whether increasing SEM was an indicator of subsequent skin damage, first observations were selected in which no Stage 2+PU damage was noted according to visual assessment and for which no incontinence was observed. Then, proportional odds models using generalized ordered logistic modeling (Stata 9, StataCorp, College Station, TX) were used to determine whether SEM measures predicted occurrence of skin damage 1 week later, controlling for within-subject clustering. Skin damage was an ordered outcome of none, erythema/Stage 1 PU, or Stage 2+PU. Covariates were Braden Pressure Sore Risk Assessment Scale score (e.g., at-risk scores of 0–18 and no-risk scores of ≥19), SEM readings concurrent with visual assessment, and ethnicity. An odds ratio (OR) was calculated for a 100-unit change in DPU reading, because 1-unit changes in DPU readings were too small to be of clinical use. The OR for 100-unit change in DPU reading was calculated using the following formula: exp(100 × β1 SEM 1 wk earlier +0 × β2 concurrent SEM+0 × β3 Braden Score risk status+0 × β4 ethnicity).
RESULTS
Skin health and SEM measures were obtained from 35 participants and are reported in this article. Table 1 presents demographic, medical, and functional characteristics of the participants for the variables most relevant to this study. The participants were predominantly non-Hispanic white (80%) females (83%) who were cognitively impaired (mean MDS recall score ± standard deviation 2.14 ± 1.15), functionally impaired (mean MDS bed mobility score 2.34 ± 3.43), and at risk for PU development (mean Braden PU Risk Score 16.54 ± 3.63). A total of 28 incident Stage 2+PUs developed in 16 subjects over the 52 weeks for a yearly incidence rate of 46% in this sample of at-risk subjects.
Table 1.
Characteristics of Study Participants (N = 35)
| Characteristic | Value |
|---|---|
| Female, % | 82.8 |
| Non-Hispanic white, % | 80 |
| Age, mean ± SD | 84.7 ±11.1 |
| Length of stay, months, mean ± SD | 29.2 ± 29.6 |
| MDS Bed Mobility Score (range 0–4), mean ± SD* | 2.3 ± 3.4 |
| MDS Transfer self-performance = total dependence, % | 71 |
| MDS Recall Score (range 0–4), mean ± SD† | 2.1 ± 1.2 |
| Braden PU risk score (range 0–23), mean ± SD‡ | 16.5 ± 3.6 |
| MDS PU Resident Assessment Protocol initiated at baseline, % | 74.2 |
0 = independent and 4 = total dependence.
0 = no recall to 4 = answers four memory questions correctly.
19–23 = no risk, < 18 = at risk.
MDS = Minimum Data Set; PU = pressure ulcer; SD = standard deviation.
Table 2 shows SEM measures for each anatomic location according to VSA outcome. There were significant differences between SEM measures and VSA outcomes, with a clear relationship showing higher concurrent SEM measures with greater skin damage. For all sites combined, mean SEM was 97 ± 122 for normal skin, 192 ± 188 for erythema/Stage 1, and 569 ± 320 for Stage 2+PUs. Pairwise post hoc differences were significant between all skin condition categories (normal—erythema/Stage 1 PU; normal—Stage 2+PU; and erythema/Stage 1 PU—Stage 2+PU; all P <.001). This relationship existed across all anatomic sites and in observations with and without incontinence present. Consistent with these findings, the upper ends of the distributions of SEM scores, as indicated by comparison of median and 75th percentile values, differentiated across skin categories more than the lower ends of the distribution. Graphically, this is presented in Figure 2, with the cumulative percentage of observations plotted according to VSA. Although SEM values ranged across the distribution for all three types of VSAs, SEM increased as skin damage severity increased. For observations with no skin damage, approximately 50% of the observations had SEM values of 50 DPU or less, compared with SEM values of approximately 150 DPU for erythema/Stage 1 and 750 DPU for Stage 2 or more.
Table 2.
Distributional Characteristics of Subepidermal Moisture Measure for All Observations and Those with No Incontinence Observed According to Visual Skin Assessment and Anatomic Site
| Anatomic Location | Visual Skin Assessment | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Erythema/Stage 1 | Stage 2 Pressure Ulcers | |||||||||||||
| N | Mean | ± SD | Median | P75 | N | Mean | ± SD | Median | P75 | N | Mean | ± SD | Median | P75 | |
| Right trochanter | |||||||||||||||
| All observations | 1,353 | 74.9 | ± 88.6 | 40 | 88 | 19 | 177.2 | ± 240.4 | 49 | 330 | 7 | 753.1 | ± 208.7 | 820 | 864 |
| No incontinence | 957 | 69.2 | ± 82.5 | 38 | 83 | 7 | 140.6 | ± 197.3 | 33 | 427 | 1 | 799.0 | — | — | — |
| Left trochanter | |||||||||||||||
| All observations | 1,361 | 73.5 | ± 83.6 | 40 | 89 | 21 | 180.9 | ± 203.6 | 61 | 319 | 0 | — | — | — | — |
| No incontinence | 959 | 66.7 | ± 78.9 | 36 | 76 | 9 | 111.4 | ± 167.1 | 43 | 61 | 0 | — | — | — | — |
| Right ischial | |||||||||||||||
| All observations | 1,081 | 143.5 | ± 165.8 | 64 | 226 | 296 | 202.7 | ± 189.1 | 157 | 300 | 10 | 483.6 | ± 357.1 | 390 | 869 |
| No incontinence | 778 | 100.1 | ± 134.4 | 41 | 143 | 188 | 156.5 | ± 170.3 | 93 | 249 | 6 | 440.5 | ± 355.6 | 298 | 869 |
| Left ischial | |||||||||||||||
| All observations | 1,089 | 139.2 | ± 162.5 | 61 | 227 | 285 | 225.1 | ± 206.0 | 195 | 316 | 9 | 329.9 | ± 231.9 | 320 | 350 |
| No incontinence | 794 | 94.2 | ± 125.4 | 36 | 133 | 170 | 175.2 | ± 191.6 | 85 | 289 | 6 | 230.7 | ± 114.3 | 241 | 320 |
| Left buttock | |||||||||||||||
| All observations | 1,147 | 80.4 | ± 102.0 | 36 | 96 | 191 | 165.2 | ± 186.1 | 108 | 240 | 45 | 638.2 | ± 314.6 | 789 | 898 |
| No incontinence | 859 | 59.7 | ± 76.2 | 31 | 66 | 88 | 119.4 | ± 172.0 | 50 | 153 | 23 | 588.5 | ± 327.3 | 728 | 896 |
| Right buttock | |||||||||||||||
| All observations | 1,152 | 81.2 | ± 97.0 | 39 | 112 | 191 | 145.1 | ± 167.8 | 86 | 212 | 42 | 543.1 | ± 325.6 | 719 | 828 |
| No incontinence | 856 | 61.4 | ± 72.4 | 32 | 69 | 94 | 121.2 | ± 156.4 | 57 | 168 | 20 | 529.8 | ± 345.0 | 722 | 829 |
| Sacral | |||||||||||||||
| All observations | 952 | 97.4 | ± 121.4 | 49 | 131 | 363 | 195.7 | ± 171.9 | 180 | 294 | 65 | 564.1 | ± 317.5 | 753 | 826 |
| No incontinence | 704 | 76.5 | ± 96.1 | 43 | 87 | 221 | 185.2 | ± 160.5 | 180 | 294 | 41 | 547.4 | ± 345.2 | 753 | 841 |
| All sites combined | |||||||||||||||
| All observations | 8,135 | 96.7 | ± 122.3 | 43 | 133 | 1,366 | 191.5 | ± 187.6 | 153 | 288 | 178 | 568.9 | ± 319.5 | 752 | 852 |
| No incontinence | 5,907 | 74.6 | ± 97.5 | 36 | 85 | 777 | 159.6 | ± 172.5 | 89 | 251 | 97 | 529.9 | ± 335.9 | 692 | 845 |
SD = standard deviation; P75 = 75th percentile.
Figure 2.
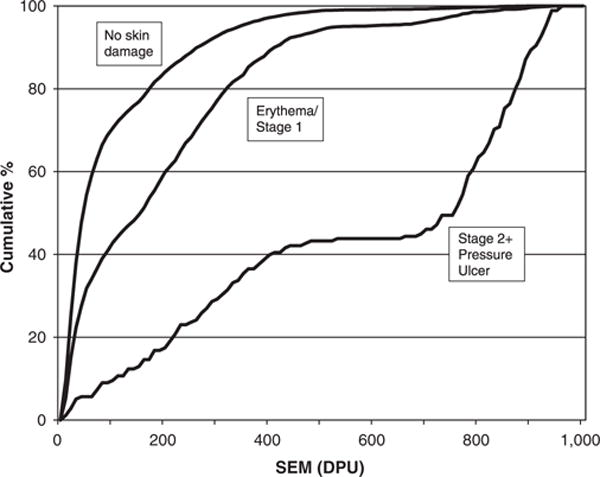
Cumulative distribution of subepidermal moisture (SEM) is provided for each of three levels of visual skin assessment (VSA): no skin damage, mild skin damage (erythema or Stage 1 pressure ulcer), and more-severe skin damage (Stage 2+pressure ulcer). When evaluated at the 50% level for cumulative frequency according to VSA, SEM values are lowest when no skin damage is observed, followed by higher values for mild damage and highest values for severe damage.
SEM measures were responsive to changes observed visually in skin condition from week to week. SEM change scores were lower for those with a visual skin change pattern of no change in level of skin damage than for those with patterns of deterioration and improvement (mean change in SEM: no change 16.14 ± 149.87, deteriorated 98.32 ± 232.14, improved − 33.47 ± 230.42). All pairwise post hoc differences were significant for sacral and right buttock sites (all P <.05, except no change vs deteriorated for right buttock).
To determine whether SEM predicted skin damage 1 week later, proportional odds models were evaluated using sacral and buttocks SEM data collected at timepoints when there was no evidence of Stage 2+PU skin damage according to visual assessment, controlling for clustering of data within subjects. Other skin locations (trochanters and ischial tuberosities) were not used in the model because of low incidence of skin damage at these sites. SEM values predicted the incidence of erythema/Stage 1 PU damage identified 1 week later (Table 3; OR = 1.26 per 100 DPU), adjusting for concurrent SEM. The OR for SEM prediction of erythema/Stage 1 damage deteriorating to Stage 2+PUs was similar but not statistically significant, perhaps because of the small number of observations that progressed to Stage 2+PUs. Analyses were also performed adjusting for known risk factors (Braden Scale total scores 1–18 vs ≥19) and ethnicity (non-Hispanic white vs other). When only risk status or ethnicity was added alone to the model, results were similar to the unadjusted model (data not shown), with risk (at risk) or ethnicity (other) significantly related to subsequent skin damage. When both were added to the model, results were similar, but SEM was no longer significant (Table 3).
Table 3.
Predicted Skin Damage 1 Week Later Using Subepidermal Moisture (SEM) Measures with Models Unadjusted and Adjusted for Ethnicity and Risk
| Independent Variable | Subsequent Visual Skin Assessment (1 Week Later) | |||
|---|---|---|---|---|
| Unadjusted Model | Adjusted Model | |||
| None Versus Erythema/Stage 1 PU | None or Erythema/Stage 1 PU Versus Stage 2+PU | None Versus Erythema/Stage 1 PU | None or Erythema/Stage 1 PU Versus Stage 2+PU | |
| OR (99% Confidence Interval) | ||||
| SEM | 1.003 (0.999–1.005)* | 1.003 (0.998–1.007) | 1.002 (0.999–1.005) | 1.002 (0.996–1.008) |
| Subsequent SEM (1 week later) | 1.005 (1.003–1.007)* | 1.008 (1.005–1.011)* | 1.004 (1.003–1.006)* | 1.008 (1.004–1.012)* |
| Anatomic location (reference = sacral) | ||||
| Left buttock | 0.353 (0.159–0.785)* | (0.235 0.008–6.594) | 0.323 (0.145–0.719)* | 0.240 (0.009–6.668) |
| Right buttock | 0.867 (0.421–1.785) | 2.224 (0.252–19.638) | 0.798 (0.373–1.709) | 1.916 (0.203–18.060) |
| Non-Hispanic white ethnicity | 3.851 (1.254–11.831)* | 1.241 (0.100—15.387) | ||
| At risk for pressure ulcers (Braden score≤18) | 2.322 (0.750–7.187) | 6.775 (0.635–72.258)* | ||
| 100-unit change in the SEM measure | 1.256 | 1.216 | ||
P ≤.05; Proportional odds models using generalized logistic regression. Odds ratios (ORs) reflect a 1-unit change in the SEM measure. Excludes observations with incontinence or Stage 2 + pressure ulcer (PU) present during initial SEM measure.
DISCUSSION
In this sample of NH residents, it was found that higher SEM was associated with concurrent and incident (1 week later) skin damage. The concurrent SEM measures were associated with level of skin damage across all anatomic locations, with and without incontinence. The SEM measure was responsive to changes in VSA, with SEM measures increasing significantly as visual skin damage increased, remaining stable with no skin change, and decreasing as existing skin damage improved. This supports earlier case studies of SEM and wound healing. One study used SEM measures to compare healing of split-thickness autografts with healing of cultured skin substitutes in five patients with paired site comparisons. It showed that SEM values decreased as sites healed and epidermis matured.25 SEM values approached values for uninjured skin by 12 days.25 Similarly, the current study found decreases in SEM values associated with skin damage improvement.
It was found that SEM measures predicted erythema and Stage 1 PU skin damage visible 1 week later at the sacrum and buttocks locations. The SEM values measured using the dermal phase meter were higher when there was no visible skin damage at the time but skin damage (e.g., erythema/Stage 1 PU or Stage 2+PU) developed and was visible on the skin the next week. The OR per 100-unit change in DPU of 1.26 indicated that the values obtained using the dermal phase meter predicted 26% of the erythema/Stage 1 PUs that developed and were visible on the skin the following week in the study sample. The SEM device can be used for multiple patients and is a small, durable handheld unit the size of a personal digital assistant (PDA), with a 6″ by 1″ wand attached by a cord that fits in a laboratory coat pocket and displays the SEM value immediately after a light, 5-second skin touch. As such, if the findings from this study are replicated in a larger sample in which it is possible to determine more-specific cutpoints, the device would be practical and applicable for clinical settings, much like a blood glucose monitor. The ability to detect PUs before visible skin damage would allow for earlier initiation of prevention interventions such as scheduled repositioning, use of pressure reduction support surfaces, and skin protection with topical preparations.
There are several limitations of the study. First, although ethnicity was a strong predictor in the model, it was not possible to evaluate SEM and early skin damage in persons with darkly pigmented skin because of the small number of subjects with darkly pigmented skin. Others have reported differences in detection of early skin damage in persons with darkly pigmented skin.4,32,33 Second, ulcers on the heels, ischium, or trochanters were not evaluated because of infrequent occurrence of skin damage or (for ischium) potential confounding by incontinence at these sites in this NH sample. Finally, this was a small sample, yet the intervention was time intensive because of the frequency of the skin assessments performed (weekly) for 1 year. These findings need to be replicated in a larger sample.
The study of SEM in PU development is new, and factors influencing variability in measurement are still emerging, which may obscure differences in SEM changes that indicate the early stages of skin damage. However, even when other single factors in the model were controlled for, SEM remained a significant predictor of skin damage. Translating SEM values into clinically meaningful indicators shows promise, even with the wide distributions of measures seen. The cumulative distribution frequency showed that SEM demonstrates increased differentiation between skin health categories at higher values and less differentiation between skin health categories at lower values. It may be that persons are being identified as positive for erythema/Stage 1 development who do not develop damage because of the variability in the SEM measures. However, the measure identifies those who may benefit from early aggressive intervention as opposed to missing them. SEM values may be useful to monitor for impending skin damage and as a future method of targeting prevention interventions.
Acknowledgments
We thank Emmett Keeler, PhD, for thoughtful contributions to the data analysis plan and for suggestions on early drafts of the manuscript and NOVA Technology Corporation, Portsmouth, New Hampshire, for providing dermal phase meters.
Financial Disclosure: This research was supported by grants from the National Institute on Aging: UCLA Claude D. Pepper Older Americans Independence Center Pilot Grant, and Sigma Theta Tau Nursing Honor Society, UCLA Chapter.
Sponsor’s Role: The sponsor had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the manuscript.
Footnotes
Author Contributions: Barbara M. Bates-Jensen: conception and design of study, acquisition of subjects, statistical analysis, analysis and interpretation of data, and preparation of manuscript. Heather E. McCreath: statistical analysis, analysis and interpretation of data, and preparation of manuscript. Ayumi Kono: statistical analysis; collection, analysis, and interpretation of data; preparation of manuscript. Neil Christopher R. Apeles: data collection, review of draft manuscripts. Cathy Alessi: overall guidance on study design, data interpretation, and review of final manuscript.
References
- 1.Bergstrom N, Braden B. A prospective study of pressure sore risk among institutionalized elderly. J Am Geriatr Soc. 1992;40:747–758. doi: 10.1111/j.1532-5415.1992.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 2.Berlowitz DR, Bezerra HQ, Brandeis GH, et al. Are we improving the quality of nursing home care: The case of pressure ulcers. J Am Geriatr Soc. 2000;48:59–62. doi: 10.1111/j.1532-5415.2000.tb03029.x. [DOI] [PubMed] [Google Scholar]
- 3.Meehan M. National pressure ulcer prevalence survey. Adv Wound Care. 1994;3:27–30. [PubMed] [Google Scholar]
- 4.Ambung SR, Miller WL, Bosley LM. The 1999 National Pressure Ulcer Prevalence Survey: A benchmarking approach. Adv Skin Wound Care. 2001;14:297–301. doi: 10.1097/00129334-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Halfens RJG, Bours GJJW, van Ast W. Relevance of the diagnosis ‘Stage 1 pressure ulcer’: An empirical study of the clinical course of Stage 1 ulcers in acute care and long-term hospital populations. J Clin Nurs. 2001;10:748–757. doi: 10.1046/j.1365-2702.2001.00544.x. [DOI] [PubMed] [Google Scholar]
- 6.Allman RM, Goode PS, Patrick MM, et al. Pressure ulcer risk factors among hospitalized patients with activity limitation. JAMA. 1995;273:865–870. [PubMed] [Google Scholar]
- 7.Ek A. Prevention, treatment, and healing of pressure sores in long-term care patients. Scand J Caring Sci. 1987;1:7–13. doi: 10.1111/j.1471-6712.1987.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 8.Schnelle JF, Adamson GM, Cruise PA, et al. Skin disorders and moisture in incontinent nursing home residents: Intervention implications. J Am Geriatr Soc. 1997;45:1182–1188. doi: 10.1111/j.1532-5415.1997.tb03767.x. [DOI] [PubMed] [Google Scholar]
- 9.Bergstrom N, Braden B, Kemp M, et al. Predicting pressure ulcer risk: A multisite study of the predictive validity of the Braden Scale. Nurs Res. 1998;47:261–269. doi: 10.1097/00006199-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Bergstrom N, Braden B, Kemp M, et al. Multi-site study of incidence of pressure ulcers and the relationship between risk level, demographic characteristics, diagnoses, and prescription of preventive interventions. J Am Geriatr Soc. 1996;44:22–30. doi: 10.1111/j.1532-5415.1996.tb05633.x. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Practice Guideline Number 3. Rockville, MD: Agency for Health Care Policy and Research; US Department of Health and Human Services; 1992. Panel for the prediction and prevention of pressure ulcers in adults. Pressure Ulcers in Adults: Prediction and Prevention. (Publication no. AHCPR 92-0047). [Google Scholar]
- 12.NPUAP Revised Stage 1 pressure ulcer definition [on-line] Available at http://www.npuap.org/positn4.htm Accessed April 23, 2007.
- 13.Meraviglia M, Becker H, Grobe SJ, et al. Maintenance of skin integrity as a clinical indicator of nursing care. Adv Skin Wound Care. 2002;15:24–29. doi: 10.1097/00129334-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Russell JA, Lichtenstein SL. Randomized controlled trial to determine the safety and efficacy of a multi-cell pulsating dynamic mattress system in the prevention of pressure ulcers in patients undergoing cardiovascular surgery. Ostomy Wound Manage. 2000;46:46–55. [PubMed] [Google Scholar]
- 15.Clever K, Smith G, Bowser C, et al. Evaluating the efficacy of a uniquely delivered skin protectant and its effect on the formation of sacral/buttocks pressure ulcers. Ostomy Wound Manage. 2002;48:60–67. [PubMed] [Google Scholar]
- 16.Sprigle S, Linden M, Riordan B. Analysis of localized erythema using clinical indicators and spectroscopy. Ostomy Wound Manage. 2003;49:42–52. [PubMed] [Google Scholar]
- 17.Schubert V, Fagrell B. Local skin pressure and its effects on skin microcirculation as evaluated by laser-Doppler fluxemetry. Clin Physiol. 1989;9:535–545. doi: 10.1111/j.1475-097x.1989.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 18.Herrman EC, Knapp CF, Donofrio JC, et al. Skin perfusion responses to surface pressure-induced ischemia: Implications for the developing pressure ulcer. J Rehabil Res Dev. 1999;36:109–120. [PubMed] [Google Scholar]
- 19.Pack R, Chang DS, Brevetti LS, et al. Correlation of a simple direct measurement of muscle pO2 to a clinical ischemia index and histology in a rat model for chronic severe hindlimb ischemia. J Vasc Surg. 2002;36:172–179. doi: 10.1067/mva.2002.124361. [DOI] [PubMed] [Google Scholar]
- 20.Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27:2642–2647. doi: 10.2337/diacare.27.11.2642. [DOI] [PubMed] [Google Scholar]
- 21.Chambers MA, Jahans K, Whelan A, et al. Simple objective measurement of the cutaneous delayed-type hypersensitivity reaction to tuberculin using spectrophotometry. Skin Res Technol. 2002;8:89–93. doi: 10.1034/j.1600-0846.2001.80205.x. [DOI] [PubMed] [Google Scholar]
- 22.Palensek J, Morhenn VB. Changes in the skin’s capacitance after damage to the stratum corneum in humans. J Cutan Med Surg. 1999;3:127–131. doi: 10.1177/120347549900300304. [DOI] [PubMed] [Google Scholar]
- 23.Boyce ST, Supp AP, Harriger MD, et al. Surface electrical capacitance as a noninvasive index of epidermal barrier in cultured skin substitutes in athymic mice. J Invest Dermatol. 1996;107:82–87. doi: 10.1111/1523-1747.ep12298286. [DOI] [PubMed] [Google Scholar]
- 24.Ho DQ, Bello YM, Grove GL, et al. A pilot study of noninvasive methods to assess healed acute and chronic wounds. Dermatol Surg. 2000;26:42–49. doi: 10.1046/j.1524-4725.2000.99143.x. [DOI] [PubMed] [Google Scholar]
- 25.Goretsky MJ, Supp AP, Greenhalgh DG, et al. Surface electrical capacitance as an index of epidermal barrier properties of composite skin substitutes and skin autografts. Wound Rep Reg. 1995;3:419–425. doi: 10.1046/j.1524-475X.1995.30406.x. [DOI] [PubMed] [Google Scholar]
- 26.Harrow JJ, Mayrovitz HN. Initial assessment of tissue water content surrounding pressure ulcers in spinal cord injury patients. Abstract. Symposium on Advance Wound Care Medical Research Forum on Wound Repair; April 2006. [Google Scholar]
- 27.Braden BJ, Bergstrom N. A conceptual schema for the study of etiology of pressure sores. Rehabil Nurs. 1987;12:8–12. doi: 10.1002/j.2048-7940.1987.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 28.Bergstrom N, Demuth PJ, Braden BJ. A clinical trial of the Braden Scale for predicting pressure sore risk. Nurs Clin North Am. 1987;22:417–428. [PubMed] [Google Scholar]
- 29.Maklebust J. Pressure ulcer staging systems: NPUAP Conference Proceedings. Adv Wound Care. 1995;8:2811–2814. [PubMed] [Google Scholar]
- 30.Blank IH, Maloney J, III, Emslie AG, et al. The diffusion of water across the stratum corneum as a function of its water content. J Invest Dermatol. 1984;82:188–194. doi: 10.1111/1523-1747.ep12259835. [DOI] [PubMed] [Google Scholar]
- 31.Tagami H, Ohi M, Iwasuki K, et al. Evaluation of the skin surface hydration in vivo by electrical measurement. J Invest Dermatol. 1980;75:500–507. doi: 10.1111/1523-1747.ep12524316. [DOI] [PubMed] [Google Scholar]
- 32.Lyder C, Preston J, Scinto J, et al. Final Report. Washington, DC: U.S. Health Care Financing Administration; 1998. Medicare Quality Indicator System: Pressure Ulcer Prediction and Prevention Module. [Google Scholar]
- 33.Cuddigan J, Ayello E, Sussman C, editors. Pressure Ulcers in America: Prevalence, Incidence, and Implications for the Future. Reston, VA: National Pressure Ulcer Advisory Panel; 2001. [DOI] [PubMed] [Google Scholar]


