Abstract
Background and purpose
Anti-NMDA receptor encephalitis is an autoimmune disease mediated by IgG1 or IgG3 antibodies to the GluN1 subunit of the NMDAR, resulting in down-regulation of NMDA receptors. Early diagnosis, prompt reduction of anti-NMDAR antibodies and removal of associated ovarian tumors when identified are important drivers of prognosis.
Materials and methods
Immunohistochemical studies were performed to evaluate B-cell, plasma cell, and T-cell infiltrates in the brain of a 3-year-old patient with anti-NMDA receptor encephalitis who failed to improve after plasma exchange and Rituximab treatment. Complement activation was evaluated by C4d staining.
Results
Plasma cells and B-cells were rarely detected in the brain. In contrast, persistent intra-parenchymal infiltrates and perivascular CD3+ T-cells and evidence of complement activation were detected. Activated microglia and microglial nodules were also detected in the frontal lobes and basal ganglia.
Conclusion
The role of T-cells and complement activation should be investigated in patients who do not respond to plasma exchange and Rituximab treatment.
Keywords: T-cells, NMDAR, NMDA receptor encephalitis, autoimmune encephalitis, complement activation
INTRODUCTION
Since its discovery in 2007, over 600 worldwide cases of the autoimmune encephalitis disease associated with antibodies against the N-methyl-D-aspartate receptor (NMDAR) have been documented in the literature1–5. NMDAR autoimmune encephalitis (NMDAR-AE) is the most common form of autoimmune encephalitis6–8, and is associated with a set of psychiatric and neurological symptoms with rapid onset9,10. Hospitalization of NMDAR-AE patients averages 3–4 months with mean recovery time following first-line treatment of 14 months although 20–25% of patients will have a disease relapse11. Prognosis in NMDAR-AE is dependent on early recognition, prompt reduction of anti-NMDAR antibodies, and removal of associated tumors when identified1,4.
The condition is often associated with CSF lymphocytic pleocytosis, olygoclonal bands, and a frequently unremarkable brain MRI12. High titer detection of IgG antibodies to the GluN1 subunit of the NMDAR (also known as NR1) in serum or CSF confirms the diagnosis7, and high titers of CSF antibody in particular are associated with worse outcome3,11, most likely due the ability of the anti-NMDAR antibodies to cause a reversible decrease in NMDA receptors on neuronal surfaces13. Females with ovarian teratomas are highly susceptible to NMDAR-AE since the tumor contains a component of immature neural tissue expressing NMDA receptors, allowing the immune system access to this self-antigen without the protection of the blood brain barrier. Thus, removal of the teratoma is a first-line therapy in such cases. Glucocorticoids, intravenous immune globulin, and plasma exchange aimed at inhibiting the B-cell mediated immune response have also been used, but in cases where first-line treatment fails, Rituximab and cyclophosphamide have also been given12.
Histopathologic examination of post-mortem brain samples of NMDAR-AE cases have revealed microglial activation and perivascular B-cell and plasma cell infiltrates14. Rare perivascular and intra-parenchymal T-cells have also been reported, however, the role of T-cells in the pathogenesis of this condition has not been investigated to date, particularly in cases that are refractory to treatment13,15. While IgG1 and IgG3 subtypes can potentially activate complement, it remains unknown whether complement activation plays a significant role in pathogenesis of NMDAR-AE, particularly in those cases that are refractory to treatment13.
In this case report, the NMDAR-AE patient failed to respond to standard therapy with plasma exchange and rituximab. At autopsy, CD20+ B cells were rare as expected since this patient had recently been treated with Rituximab, as were CD138+ plasma cells, but perivascular and parenchymal CD4+ and CD8+ T-cell infiltrates were persistently detected. Complement activation as detected by C4d staining was also evident. We conclude that the role of T-cells and complement activation in patients who don’t respond to standard treatment needs to be investigated in a larger cohort of NMDAR-AE cases.
METHODS
Tissue selection
The whole brain was sectioned in the coronal plane after fixation in 20% formalin for a period of two weeks. Once sectioned, >20 brain regions were selected for H&E histology including gray and white matter from the bilateral frontal, temporal, parietal, and occipital lobes, bilateral basal ganglia, bilateral hippocampi, and brain stem, based on a modified protocol for pediatric autopsy at our institution.
Immunohistochemistry
Four micron thick sections of formalin-fixed, paraffin-embedded tissue underwent heat-induced epitope retrieval using CC1 (Ventana Medical Systems, Inc., Tucson, Arizona, USA), a Tris-based buffer at pH 8–8.5, followed by IHC staining with rabbit monoclonal CD3 (Ventana), mouse monoclonal CD4 (Leica Biosystems, Ltd., Newcastle, United Kingdom), rabbit monoclonal CD8 (Ventana), mouse monoclonal CD20 (Ventana), mouse monoclonal CD68 (Dako, Carpinteria, CA, USA), mouse monoclonal CD138 (Dako), mouse monoclonal HLA-II (anti-HLA-DP, -DQ, -DR) (Dako), or mouse monoclonal C4d (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Microscopy
Photographs were taken with a Nikon DS-Fi1-L2 microscope camera (Nikon Corporation, Melville, NY, USA) using ACT-1 software Version 2.63 (Nikon). Cell counts for CD4+ and CD8+ T-lymphocytes were performed manually from images of 10 identical regions of the bilateral frontal neocortex and basal ganglia.
CASE REPORT
This 3-year-old male child presented with a history of seizures, chorea and agitation prompting an MRI, which was read as normal. There was no reported history of a preceding flu-like illness11. Anti-NMDA receptor IgG was measured in both the serum and CSF at the time of presentation, demonstrating a titer of 1:2,560 and 1:160, respectively. Based on these serological findings, the patient was given a clinical diagnosis of anti-NMDA receptor autoimmune encephalitis.
The patient was treated with corticosteroids, plasma exchange and then Rituximab, with some improvement. However, his agitation and frequency of seizures worsened and he was admitted for further workup and further therapy. During the admission, the patient became pulseless and apneic and could not be resuscitated. At autopsy, the immediate cause of death was determined to be aspiration pneumonia.
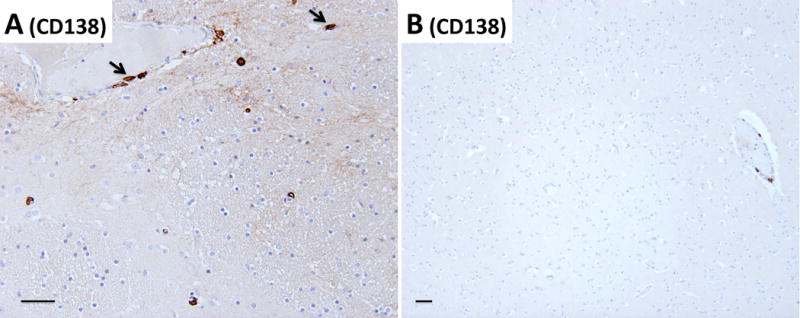
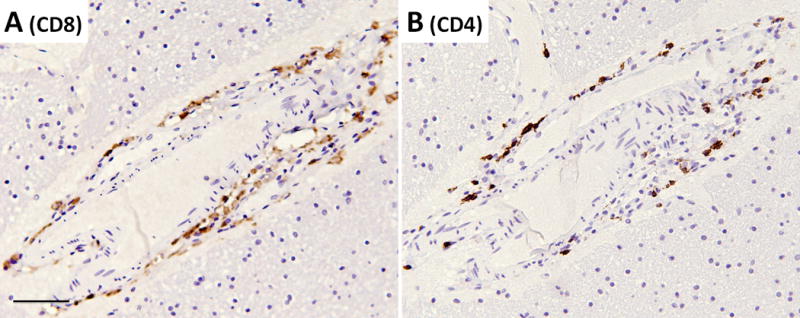
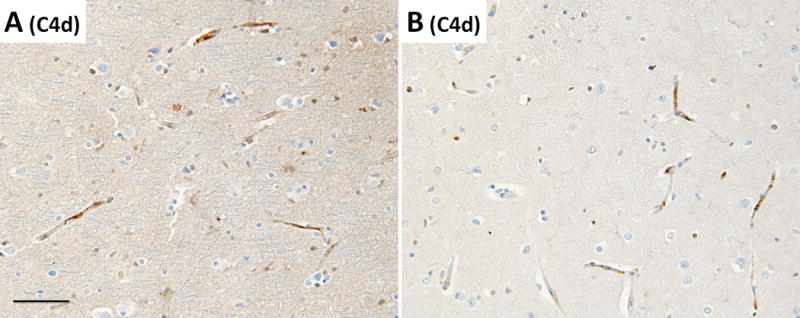
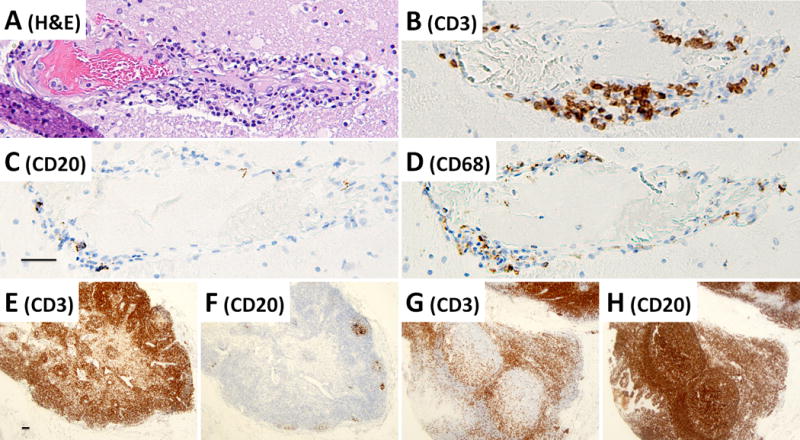
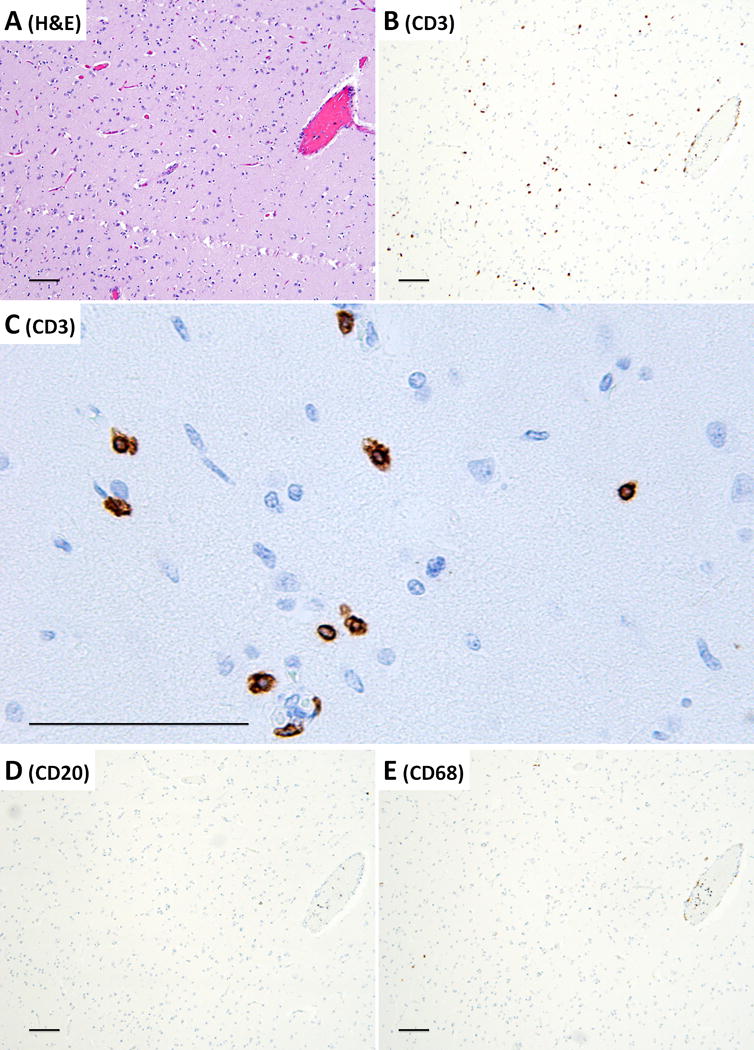
Detailed examination of the brain at autopsy showed microglial activation and microglial nodule formation by CD68 and HLA-II (Figure 1A–C) that were particularly prominent in basal ganglia, as well as perivascular mononuclear cell infiltrates by H&E (Figures 2–3). GFAP showed astrocytes that were consistent with reactive gliosis (data not shown). Parenchyma near vessels within the basal ganglia were particularly inundated with mononuclear infiltrates by H&E (Figure 2A) which consisted primarily of CD3+ T cells (Figure 2B). As expected, few CD20+ B cells (Figure 2C) were detected in this patient who had recently received Rituximab treatment. A thoracic lymph node from this patient also demonstrated prominent CD3+ T cells (Figure 2E), but few CD20+ B cells (Figure 2F) in comparison to lymph node tissue from a patient who had not been treated with Rituximab (Figure 2G and 2H). CD3+ T cells were also detected in the parenchyma of the frontal lobe (Figure 3B–C). B-cells (Figure 3D) and plasma cells (Figure 4) were very rare compared to previous reports. Both the basal ganglia and frontal lobe tissues displayed a CD4:CD8 T cell ratio of approximately 1:1 (Figure 5). C4d immunostaining highlights granular endothelial staining in small vessels of both the frontal lobe and basal ganglia (Figure 6).
Figure 1.
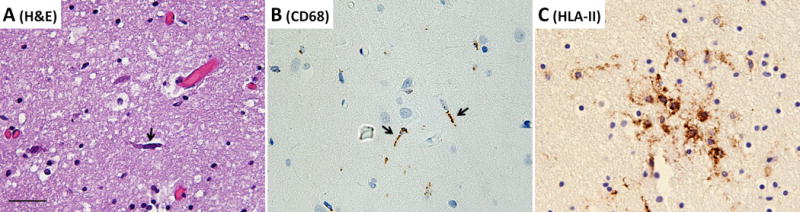
(A) H&E, (B) CD68, and (C) HLA-II immunostaining showing activated microglia (arrows) and a microglial nodule in the basal ganglia. All images taken at a total of 200× magnification, scale bar = 50μm.
Figure 2.
A basal ganglia vessel demonstrating residual perivascular T-lymphocyte cuffing after Rituximab therapy. (A) H&E section, (B) CD3 immunohistochemistry to detect perivascular T-cells, (C) CD20 immunohistochemistry to detect B-cells, and (D) CD68 immunohistochemistry to detect histiocytes. As a control, a thoracic lymph node from the Rituximab-treated NMDAR-AE patient was used to detect the presence of (E) CD3-immunopositive T-cells and (F) CD20-immunopositive B-cells as compared to a lymph node from a patient without Rituximab treatment (G–H). Images A–D are taken at a total of 100× magnification, images E–H are taken at a total magnification of 20×, all scale bars = 50μm.
Figure 3.
Frontal lobe parenchyma demonstrating perivascular CD3+ T-lymphocyte cuffing and parenchymal CD3+ T-lymphocyte infiltration. (A) H&E section, (B) CD3 immunohistochemistry to detect T-cells, (C) CD3 immunohistochemistry at higher power, demonstrating T-cell morphology in the brain parenchyma (D) CD20 immunohistochemistry to detect B-cells, and (E) CD68 immunohistochemistry to detect histiocytes. Images A, B, D, and E are taken at a total of 40× magnification, image C is taken at a total of 200× magnification, all scale bars = 100μm.
Figure 4.
CD138-staining demonstrating rare plasma cells around vessels and in the parenchyma of (A) the basal ganglia and (B) frontal lobe. Arrows rare identify examples of CD138+ plasma cells. Pictures are taken at a total magnification of 100× (A) and 40× (B), scale bar = 50μm.
Figure 5.
T-cell immunohistochemistry in a frontal lobe blood vessel showing perivascular T-lymphocyte cuffing with approximately equal distribution between (A) CD8 T-cells and (B) CD4 T-cells. All pictures taken at a total of 100× magnification, scale bar = 50μm.
Figure 6.
C4d immunohistochemistry showing complement deposition in the (A) frontal lobe and (B) basal ganglia. All images taken at a total of 100× magnification, scale bar = 50μm.
DISCUSSION
NMDAR-AE is a recently defined entity that is increasingly diagnosed as the cause of immune-mediated encephalitis. Often occurring in patients with a teratoma or characterized by a preceding prodromal illness, early diagnosis and aggressive treatment with a first-line regimen of glucocorticoids, intravenous immune globulin, and plasma exchange and second-line treatment of Rituximab or Cyclophosphamide, has a good prognosis in the vast majority of cases11. The main findings of this case report are microglial activation and nodule formation (Figure 1), low frequency of CD20+ B-cells, persistent parenchymal and perivascular CD3+ T-cell infiltrates (Figures 2, 3, and 5), and C4d complement activation in the frontal lobes and basal ganglia (Figure 6) in a patient who did not clinically respond to standard treatment.
Previous histopathologic studies demonstrated CD20+ B-cells dominate the perivascular and parenchymal infiltrates in brain tissue of NMDAR-AE patients13,15. In contrast, we observed a low frequency of CD20+ B-cells in the lymph node and brain tissue of the patient we report here. This was an expected finding since the patient in this case had recently received Rituximab treatment. Previous histopathogic studies also demonstrated that plasma cells which secrete antibody are also detected in the brain tissue of NMDAR-AE patients13,15. In contrast, we detected very few plasma cells in the brain tissue of the patient we report here. It is thought that activated CD20+ B-cells can cross the intact blood brain barrier and differentiate into plasma cells which produce antibodies that are detected in CSF, and persist for a prolonged period of time. It is possible that by depleting the CD20+ B cells, the plasma cell population was also reduced.
The patient in this study was treated with both Rituximab to deplete CD20+ B cells and plasma exchange to remove anti-NMDAR antibodies, and yet the patient failed to improve clinically. We did not test for anti-NMDAR antibody titer in the serum or CSF of this patient post-mortem, nor do we have a pre-treatment brain biopsy sample to establish whether CD20+ B cells dominated the patient’s mononuclear infiltrate population prior to treatment. However, we did observe that following Rituximab treatment, CD3+ T cells dominated the parenchymal mononuclear infiltrates within the brain of this patient. Complement activation near the capillaries of the parenchyma was also detected. These observations may suggest that CD3+ T cell infiltrates and complement activation may provide an alternative pathophysiological mechanism in NMDAR-AE patients that are refractory to plasma exchange and Rituximab treatment. Additional studies on the potential pathogenic role of these T-cells as well as the contribution of complement activation in NMDAR-AE are needed.
Acknowledgments
N.L.M. is supported by a grant from the National Institutes of Health (NS098229).
Footnotes
DECLARATION OF INTEREST: The authors declare no conflict of interest.
References
- 1.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54(7):899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesan A, Benavides DR. Autoimmune encephalitis and its relation to infection. Curr Neurol Neurosci Rep. 2015;15(3):3. doi: 10.1007/s11910-015-0529-1. [DOI] [PubMed] [Google Scholar]
- 6.Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 7.Pruss H, Dalmau J, Harms L, Holtje M, Ahnert-Hilger G, Borowski K, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75(19):1735–1739. doi: 10.1212/WNL.0b013e3181fc2a06. [DOI] [PubMed] [Google Scholar]
- 8.Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology. 2015;84(4):359–366. doi: 10.1212/WNL.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 9.Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurol. 2013;70(9):1133–1139. doi: 10.1001/jamaneurol.2013.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright S, Hacohen Y, Jacobson L, Agrawal S, Gupta R, Philip S, et al. N-methyl-D-aspartate receptor antibody-mediated neurological disease: results of a UK-based surveillance study in children. Arch Dis Child. 2015;100(6):521–526. doi: 10.1136/archdischild-2014-306795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. The Lancet Neurology. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77(6):589–593. doi: 10.1212/WNL.0b013e318228c136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruss H, Leubner J, Wenke NK, Czirjak GA, Szentiks CA, Greenwood AD. Anti-NMDA Receptor Encephalitis in the Polar Bear (Ursus maritimus) Knut. Scientific reports. 2015;5:12805. doi: 10.1038/srep12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camdessanche JP, Streichenberger N, Cavillon G, Rogemond V, Jousserand G, Honnorat J, et al. Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur J Neurol. 2011;18(6):929–931. doi: 10.1111/j.1468-1331.2010.03180.x. [DOI] [PubMed] [Google Scholar]


