Abstract
Objective
Evaluating cardiovascular (CVD) risk in children and youth 13–24 years old who are facing a lifetime exposure to both HIV and antiretroviral therapy (ART) is a research priority. This study compares endothelial function measured by Peripheral Arterial Tonometry in HIV+ youth infected perinatally and behaviorally as well as HIV- controls.
Methods
Three groups of participants aged 8–30 yo were enrolled; HIV+ perinatally-infected, HIV+ behaviorally-infected on ART with HIV-1 RNA <1,000 copies/mL, and HIV- controls. We measured the reactive hyperemic index (RHI), a measure of endothelial function, using endoPAT. Markers of systemic inflammation, monocyte activation, and gut integrity were also assessed. Spearman correlations and regression analyses were used to explore relationships between endothelial function measures and other measured variables.
Results
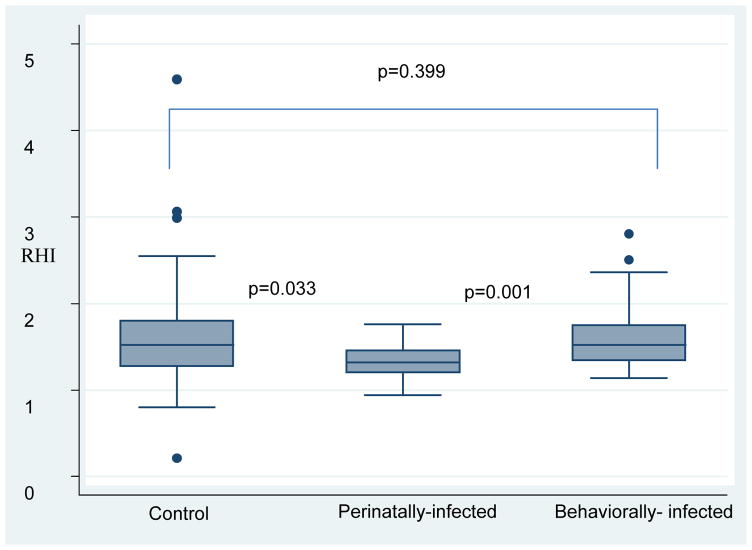
Overall, 119 participants were enrolled: 53 HIV +behaviorally infected, 18 HIV+ perinatally-infected, and 48 controls. Overall, 71 % were men; 77% African Americans and median age was 22 years old. Median (interquartile range) RHI was lower in the HIV+ perinatally-infected group [1.34 (1.20, 1.42)], compared to the behaviorally infected group [1.52 (1.34, 1.75)] and the control group [1.52 (1.27, 1.80) (p<0.01)]. Soluble CD14, a marker of monocyte activation, intestinal fatty acid-binding protein, a marker of gut integrity and soluble vascular cell adhesion molecule, a marker of vascular dysfunction, were different among the 3 groups (p≤0.01).
Conclusions
HIV+ youth infected perinatally appear to have higher levels of endothelial dysfunction and immune activation when compared to behaviorally-infected youth. Further longitudinal studies are needed to determine whether perinatally-infected youth have higher risks of CVD.
Keywords: endothelial function, perinatally-infected, behaviorally-infected, pediatric HIV, inflammation, cardiovascular disease
Introduction
Older children and adolescents now make up the largest percentage of HIV-infected children cared for at pediatric HIV clinics in the USA. The CDC estimates that 26% of the 50,000 new HIV infections diagnosed in 2010 were among youth 13–24 years old. As, cardiovascular and metabolic diseases have become the leading cause of death in HIV-infected individuals[1–4], the evaluation of CVD risk in youth who are facing a lifetime of exposure to both HIV and ART is imperative. Development of studies in this population has been hampered by lack of agreement on clinically significant and relevant outcomes. Increasingly, data in non-HIV adult and pediatric populations have shown that surrogate markers of cardiovascular disease risk, such as carotid intima-medial thickness (IMT), pulse wave velocity (PWV) and forearm flow-mediated vasodilation (FMD), are predictive of hard outcomes, such as myocardial infarction and strokes [5–8].
Changes in the endothelium are one of the earliest alterations of the vessel wall which occur prior to atherosclerosis[9]. Forearm flow-mediated vasodilation (FMD) has classically been used to assess peripheral endothelial dysfunction[10] and is predictive of long term cardiovascular events[8]. Its results, however, can vary during measurement and FMD has poor reproducibility[11]. Peripheral Arterial Tonometry (endoPAT) is an attractive tool that measures vascular function in an automatic and non-invasive manner. It assesses vascular morphology and function by registering finger pulse volume and infrared light transmission plethysmography[12]. Reactive hyperemic index (RHI) as measured by endoPAT has been found to be lower in patients with coronary endothelial dysfunction compared with those with normal coronary endothelial function and an RHI < 1.35 was found to have a sensitivity of 80% and specificity of 85% to identify patients with coronary endothelial dysfunction [13]. In several cross sectional studies, impaired endothelial function as measured by endoPAT correlated with FMD [12, 14, 15]. In multiple longitudinal studies, a lower RHI detected by endoPAT was independently associated with a higher risk of cardiac adverse events (cardiac death, myocardial infarction)[16]. In addition, endoPAT is painless and is proven to be feasible and reproducible in adolescents[17, 18].
Only two previous studies have assessed endothelial function in HIV-infected children [19, 20]. Both studies used FMD, a technique that is very difficult to perform and interpret in children and that is not standardized in this population, thus the need to use better more feasible techniques to assess endothelial function and its correlates in HIV-infected youth.
With the use of the endoPAT technique, our objective was to compare peripheral endothelial function, for the first time, in perinatally-infected and behaviorally-infected HIV positive children and youth versus uninfected controls and to study its relationship to inflammation, immune activation and markers of gut integrity.
Methods
Study design
This study is a cross-sectional analysis from a prospective, observational cohort study evaluating and comparing the prevalence and risk factors for endothelial dysfunction in HIV-infected youths in congenitally or behaviorally infected and uninfected controls. The study was reviewed and approved by the Institutional Review Board of University Hospitals Case Medical Center, Cleveland, Ohio. Written informed consent was provided by all participants. The study is registered on clinicaltrials.gov (NCT02324179). Participants enrolled were between the ages of 8 and 30 years infected with HIV, on continuous antiretroviral therapy (ART) for at least 6 months and with HIV-1 RNA <1,000 copies/mL.
We also enrolled uninfected healthy controls age 8–30 yo. Controls were recruited from the community using IRB-approved flyers, as uninfected siblings or relatives of the HIV-infected participants, or from physician referrals. Participants were excluded if they had a history of coronary artery disease or diabetes, were pregnant or lactating, or had an active infectious or inflammatory condition which could lead to changes in inflammation markers.
Study evaluation
At study visit, participants were fasting for 12 hours. Blood draws were obtained for real time measurements of lipid profiles, glucose and insulin levels. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as described [21]. Additionally, blood was processed and plasma stored for measurement of soluble and cellular markers of immune activation and markers of systemic inflammation[22, 23]. Some labs routinely obtained in HIV-infected children as part of routine HIV care were collected from medical records from the time closest to the study visit and within the last 6 months including CD4 cell count, complete blood count with differential, HIV-1 RNA level, and comprehensive chemistry panel (including kidney and liver function tests, total protein, calcium).
Inflammation and soluble immune activation markers
Soluble markers interleukin-6 (IL-6), soluble tumor necrosis factor receptors I and II (sTNF-RI and –RII), soluble CD14 (sCD14) and soluble CD163 (sCD163)], Intestinal Fatty Acid Binding Protein (IFABP), soluble Vascular Cell Adhesion Molecule (sVCAM), Lipopolysaccharide Binding Protein (LBP), D-Dimer and oxidized LDL were measured by ELISA (R&D Systems, Minneapolis, MN; Hycult Biotech, Uden, the Netherlands; Diagnostica Stago, Parsippany, NJ; Mercodia,, Uppsala, Sweden). High sensitivity C-reactive protein (hsCRP) was determined by particle enhanced immunonephelometric assay on a BNII nephelometer (Siemens, Indianapolis, IN, USA). NT-proBNP was measured by quantitative sandwich ELISAs (Siemens Healthcare, Newark, New Jersey, USA).
Endothelial function
Endothelial function was measured using peripheral arterial tonometry. The device (Itamar Medical Ltd., Caesarea, Israel) consists of two finger probes. The probe allows the application of a constant and evenly distributed near-diastolic counter pressure within the entire probe [13, 24]. The device records finger arterial pulsatile volume changes. It captures beat-to-beat plethysmographic recording of the finger arterial pulse wave amplitude with pneumatic probes. The finger probe is connected by tubing to volume reservoirs, the pressure change signals are filtered, amplified and stored for further analysis[12]. The recordings was performed with the patient in a seated position with both hands at the same level. A blood pressure cuff was placed on the upper arm (non-dominant); the probes were placed on the finger of each hand (same finger on both hands) and continuing recording of blood volume responses from both hands was initiated. After a period of stabilization, the blood pressure cuff on the study arm was inflated to 60 mm Hg above systolic pressure for 5 min. The cuff was deflated to induce reactive hyperemia and assess PAT. A reactive hyperemia index (RHI) was generated and is the post to pre -occlusion PAT signal ratio in the occluded side normalized to the control side and corrected for baseline vascular tone. A normal index is >1.67 and abnormal is ≤ 1.67. Augmentation index (AI) is calculated from PAT pulses recorded at the baseline period and the result is further normalized to heart rate of 75 beats per minute (AI 75). Lower AI values reflect better arterial elasticity.
Statistical analysis
The major objective of this study was to compare endothelial function between groups. Secondary objectives were to compare inflammatory markers, to examine associations between demographic characteristics, HIV variables and markers of systemic inflammation, immune activation and gut integrity with endothelial function. We performed descriptive analyses on all of the covariates and outcomes of interests. We checked validity of data distributions by running frequency analyses and graphical presentations.
Demographics, clinical indices and HIV-related factors are described by groups. Median and interquartile range (IQR) are reported for continuous variables and frequency and percent for nominal variables. Baseline variables as well as endpoints were compared among groups using unpaired t-tests or Kruskal-Wallis rank test as warranted by distribution for continuous variables and by Chi-Square tests, Fisher’s Exact tests, or Pearson Exact Chi-Square tests as appropriate for categorical variables.
Spearman correlation analysis was utilized to assess the relationships between measures of endothelial function and markers of inflammation, immune activation, as well as clinically relevant demographic and HIV-related factors (continuous variables only). Because of the skewed distribution of the markers, quantile (median) regression analyses were used to compare markers between groups after adjusting for demographic variables including age, sex, body mass index and current smoking status. The results were similar when additional analyses including only HIV-infected participants with HIV viral load less than 20 copies/mL were performed. All the analyses were performed using statistical software Stata 14.0.
Results
Baseline characteristics
Seventy one HIV-infected subjects (53 behaviorally-infected and 18 perinatally-infected) and 48 controls were recruited and included in the analysis. Demographic, clinical and laboratory characteristics are described in Table 1. The perinatally-infected group was younger, had more females and fewer smokers when compared to the behaviorally-infected and control groups (p<0.01).
Table 1.
Baseline Characteristics
| Controls | HIV positive | |||
|---|---|---|---|---|
| N=48 | Perinatally-infected N=18 |
Behaviorally-infected N=53 |
P value | |
| Demographics | ||||
| Age (year) | 22 (19, 26) | 18 (12, 21) | 25 (22, 28) | 0.0001 |
| Male (%) | 66% | 33% | 56% | <0.0001 |
| Black (%) | 56% | 89% | 92% | <0.0001 |
| Current Smokers (%) | 27% | 17% | 51% | 0.0080 |
| Metabolic and cardiovascular risk factors | ||||
| BMI (kg/m2) | 25 (22, | 23 (20, 27) | 24 (20, 29) | 0.6208 |
| Waist-to-hip ratio | 0.86 (0.83, 0.92) | 0.87 (0.80, 1) | 0.86 (0.82, 0.93) | 0.9791 |
| Systolic BP(mm Hg) | 123 (114, 130) | 123 (110, 133) | 126 (118, 135) | 0.4329 |
| Diastolic BP(mm Hg) | 74 (68, 80) | 73 (69, 84) | 75 (70, 80) | 0.7467 |
| Total Cholesterol (mg/dL) | 164 (145, 181) | 175 (144, 186) | 145 (124, 161) | 0.0014 |
| LDL (mg/dL) | 91 (75, 108) | 100 (72, 111) | 78 (65, 88) | 0.0049 |
| HDL (mg/dL) | 51 (45, 63) | 52 (41, 61) | 47 (40, 55) | 0.0747 |
| Triglycerides (mg/dL) | 68 (52, 91) | 101 (67, 124) | 69 (57, 97) | 0.0366 |
| HOMA IR | 2.15 (1.27, 3.17) | 3.20 (2.2, 5.08) | 1.20 (0.93, 2.84) | 0.0010 |
| Inflammatory markers | ||||
| ProBNP (pg/mL) | 22.5 (14, 36.5) | 22 (11, 47) | 16 (11, 34) | 0.3488 |
| hsCRP (ng/mL) | 910 (415, 2835) | 1014 (168, 3334) | 1239 (574, 2528) | 0.3455 |
| IL6 (pg/mL) | 1 (0.10, 1.99) | 1.36 (1.08, 3.07) | 1.02 (0, 2.99) | 0.4239 |
| sCD14 (ng/mL) | 1402 (848, 2116) | 1832 (1634, 2343) | 1400 (1040, 1769) | 0.0138 |
| sCD163 (ng/mL) | 440 (315, 578) | 550 (406, 759) | 441 (364, 776) | 0.1062 |
| sTNFα R I (pg/mL) | 953 (825, 1087) | 894 (796, 1007.7) | 863 (745, 1001) | 0.3011 |
| sTNFα R II (pg/mL) | 2124 (1920, 2572) | 2266 (2091, 2734) | 2331 (1853, 2826) | 0.3159 |
| LPB (ng/mL) | 16885 (11684, 22255) | 16483 (11276, 23397) | 20559 (15907, 25617) | 0.1162 |
| IFABP (pg/mL) | 1778 (1052, 2354) | 1534 (1073, 2053) | 2968 (1722, 4085) | 0.0011 |
| D-Dimer (ng/mL) | 192 (109, 301) | 257 (177, 385) | 223 (152, 347) | 0.1549 |
| sVCAM (ng/mL) | 641 (553, 784) | 711 (554, 817) | 735 (618, 859) | 0.0401 |
| Oxidized LDL (mU/L) | 49747 (40754, 55908) | 52239 (44719, 66148) | 46356 (39503, 54765) | 0.2328 |
| Endopat variables | ||||
| RHI | 1.52 (1.27, 1.80) | 1.34 (1.20, 1.42) | 1.52 (1.34, 1.75) | 0.0084 |
| Log RHI | 0.44 (0.24, 0.61) | 0.27 (0.19, 0.38) | 0.42 (0.29, 0.56) | 0.0070 |
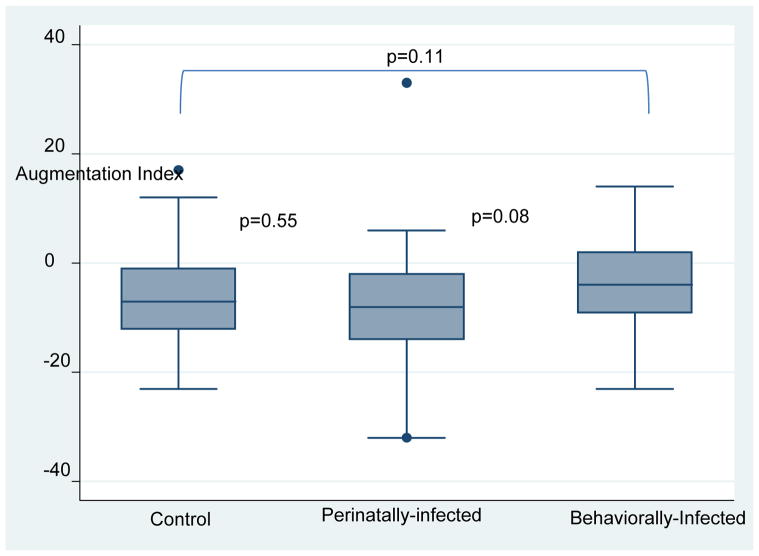
| AI | −7 (−12, −1) | −8(−14,−2) | −9 (−4, −2) | 0.1326 |
| AI 75 | −13.50 (−18, −9) | −14 (−19, −6) | −10 (−15, −6) | 0.1695 |
Data presented as median (Q1, Q3) for continuous variables and by frequency (column percent) for nominal variables.
Median BMI and waist hip ratios were similar among all 3 groups (p≤0.98). The perinatally-infected group had higher total cholesterol, LDL and triglycerides compared to the other two groups (p≤0.04). HOMA-IR was also significantly higher in the perinatally-infected group compared to the other two groups (p<0.01).
HIV characteristics
HIV-specific characteristics are described in Table 2. Perinatally-infected participants had higher CD4 cell counts [median CD4 (IQR) was 860 (735, 1173) in the perinatally-infected group vs 710 (535, 853) in the behaviorally-infected group, p=0.01]. By design all participants had viral loads less than a 1000 copies/mL and were on ART. Similar proportions of patients in both groups had viral load < 20 copies/mL (72% in the perinatally-infected group versus 77% in the behaviorally infected group; p=0.66). As expected, cumulative ART duration was longer in the perinatally-infected group [about 11.5 years for the perinatally-infected group as opposed to approximately 2.5 years in the behaviorally infected group (p<0.01)]. Both non-nucleoside reverse transcriptase inhibitor and protease inhibitor durations were longer in the perinatally-infected group (p=0.0017 and p=0.001 respectively). There was no significant difference in the proportion of participants with an AIDS diagnosis (p=0.118).
Table 2.
HIV factors
| Median | Perinatally-infected N=18 |
Behaviorally-infected N=53 |
P value |
|---|---|---|---|
| CD4 cell count (no/mm3) | 860 (735, 1173) | 710 (535, 853) | 0.0150 |
| Nadir CD4 count (no/mm3) | 473 (312, 636) | 346 (257, 550) | 0.2062 |
| HIV viral load undetectable (%) | 72% | 77% | 0.659 |
| PI (%) | 44% | 26% | 0.237 |
| Cumulative ART duration (months) | 140 (107, 197) | 29 (15, 50) | 0.0001 |
| Cumulative NNRTI duration (months) | 70 (7, 138) | 8 (0, 29) | 0.0017 |
| Cumulative PI duration (months) | 78 (15, 123) | 0 (0, 20) | 0.0001 |
| AIDS diagnosis (%) | 31% | 12% | 0.118 |
Immune activation and gut markers
Immune activation and gut markers are described in Table 1. sCD14 was highest in the perinatally-infected group (p<0.01); however I-FABP and sVCAM were highest in the behaviorally infected group (p≤0.04). All other inflammatory markers were not significantly different between groups (p≤0.42).
Endothelial function
Perinatally-infected children had lower RHI than behaviorally infected subjects and healthy controls [median (IQR) RHI level in perinatally-infected group was 1.34 (1.20, 1.42), in the behaviorally-infected group was 1.52 (1.34, 1.75), and in the control group was 1.52 (1.27, 1.80) (p<0.01)]. There was no significant difference in the augmentation index between the 3 groups (p>0.05) (see figures 1 and 2). After adjusting for age, sex, BMI and current smoking, RHI was no longer different between the groups (β≤0.59; p≤0.72) and only male sex remained significant (β=−2.05, 95% CI [−0.34–<−0.01], p=0.04). In another model, we adjusted for protease inhibitor duration and AIDS diagnosis, RHI remained significantly different between the perinatally-infected and behaviorally-infected group (β=0.26, 95% CI [0.01–0.51], p=0.04).
Figure 1.
Reactive Hyperemic Index between groups
Figure 2.
Augmentation Index between groups
Correlations with endothelial function
In univariable analyses, reactive hyperemic index correlated with age and protease inhibitor duration (p<0.01). Neither reactive hyperemic index nor augmentation index correlated with any markers of systemic inflammation, immune activation, or gut integrity (p>0.05).
DISCUSSION
This is the first study to compare endothelial dysfunction in HIV-infected children and youth infected perinatally versus behaviorally, as measured by peripheral arterial tonometry. Our results suggest that perinatally-infected, but not behaviorally-infected, youths have vascular dysfunction and possibly elevated immune activation. This extends our prior observation of increased carotid intima thickness measurements in HIV infected youth[25].
Endothelial dysfunction in HIV-infected children and youth
There are two prior studies evaluating endothelial dysfunction in HIV-infected children [19, 20]. Both studies used FMD and found it to be impaired in HIV-infected children compared to healthy controls. Bonnet et al compared HIV+ ART treated and ART naïve children and did not find any differences in the vascular variables among those two groups. On the other hand, Charakida et al found that children treated with protease inhibitors had a more pronounced FMD impairment compared to children not treated with protease inhibitors. Similarly to this report and others in HIV-infected adults[26], we found that protease inhibitors were correlated with poorer endothelial function. In our study, perinatally-infected youth have longer cumulative ART duration including NNRTI and PI duration compared to the behaviorally-infected group. However, after adjusting for both disease severity and PI duration, RHI remained significantly different between the groups. We suspect that several factors are likely contributing to impaired endothelial function in the perinatally-infected group including exposure to HIV and or ART in utero; early and long term exposure to toxic drug that have the potential to cause mitochdonrial toxicity and metabolic alterations. These findings heighten concern that sequelae of HIV infection in children and youth perinatally-infected may be more frequent and could potentially be more devastating than in behaviorally-infected youth or adults even in the setting of viral suppression.
Risk factors associated with endothelial dysfunction
Many risk factors associated with cardiovascular morbidity have also been associated with endothelial dysfunction as measured by RHI[27]. After adjusting for age, sex, body mass index and current smoking, RHI was no longer different between the groups and only male sex remained inversely related to RHI. In our cohort, the control group had twice more males than the perinatally-infected group, however the differences in RHI between the groups are unlikely due to gender imbalances alone. Several larger studies in HIV-uninfected adolescents and adults have found that men have lower hyperemic responses than women as measured by endoPAT [18, 27]. If our findings were due to gender differences alone, we would expect them to have a conservative effect on our main outcome,
We also hypothesized that RHI would be lowest in the perinatally-infected group, we were surprised to find that the control group had low median values of RHI (1.52; normal in the adult population is 1.67). Smaller studies performed in healthy and uninfected children and adolescents have reported higher RHI than our study (1.7–2.04)[17, 28]. Children in these studies were younger and the majority were Caucasians. A recent larger longitudinal study performed in healthy German children had a mean baseline RHI of 1.53[18]. Interestingly, in this same cohort, there was an interaction between RHI, age and sex; with RHI increasing with age and being consistently higher in females in all age groups. The perinatally-infected group in our study was significantly younger than the other groups which could also explain the differences observed. In addition, pubertal status has been shown to be the main predictor of microvascular function as measured by endoPAT in healthy children and adolescents, where prepubertal children had a significantly lower RHI compared with children in mid and late puberty[29]. In our analyses, RHI was correlated with age; the control group and perinatally-infected group had the largest standard deviation for age, however we did not measure pubertal status which might have influenced our findings. The relationship between age, gender and surrogate markers of CVD in HIV-infected youth is likely complex and larger study sizes are needed to detect clinically significant effects.
Endothelial dysfunction and inflammatory markers
The inflammatory markers analyzed in this study (specifically hsCRP, IL6, DDimer, NTproBNP, sVCAM, sCD14, sCD163, oxidized LDL) were selected because of their documented association with CVD and or overall morality in HIV-infected adults [30–38], however, to our knowledge only few studies have examined the relationship between markers of CVD such as cIMT and inflammation in HIV-infected youth[39, 40]. The other markers: LBP and IFAB were selected because they have been found to be elevated in HIV disease and have been used to correlate intestinal impairment and microbial translocation and inflammation in HIV[41]. Our objective in studying these markers in HIV-infected youth was to clarify the specific role of HIV infection and immune activation and markers of microbial translocation on the vascular process in the setting of lack (or few) traditional CVD risk factors. sVCAM, sCD14 and IFAB were significantly different between the groups, however none of the studied markers were associated with RHI or AI. These findings could be either because there is truly no association between any of the markers and surrogate endothelial function outcome or we failed to find any correlation because our study was not primarily designed or powered to detect an association. Further studies will be needed to ascertain the role of immune activation on cardiovascular disease risks in HIV-infected youths and more importantly to assess the progression of markers of CVD and inflammation and immune activation in this population.
Augmentation index
Despite the lower RHI, we did not find any significant differences in augmentation index between the groups. The augmentation index is defined as the percentage of the central pulse pressure attributed to the reflected pulse wave and is used as a surrogate of arterial stiffness. The augmentation index has been associated with increased cardiovascular risk scores[42], increased risk of coronary artery disease[43, 44], and all caused mortality in various patient populations[45]. We only found one other report on arterial stiffness as measured by augmentation index measurements in HIV-infected children. Kuilder et al measured arterial stiffness by pulse wave velocity and aortic augmentation index and compared 51 HIV-infected children to 52 healthy children[29]. Augmentation index was higher by 9% in HIV-infected children. Given the difference in methodology and demographic characteristics of the children in this study (mean age was 8 years, living in low-to-middle income country with a mean viral load of 34,000 copies/mL), the results are difficult to compare.
Study limitations
The small sample size and cross-sectional nature of the study are limitations. We cannot establish causation between risk factors and vascular function; however, our findings support a link between vertical transmission and lower hyperemic response. In addition our study was predominantly black youths therefore our findings cannot be generalized to different populations.
Conclusion
To our knowledge, this is the first study to investigate endothelial dysfunction in HIV positive youth perinatally vs behaviorally-infected as measured by peripheral arterial tonometry. Our results show that measuring endothelial dysfunction and arterial stiffness in this population is feasible and that perinatally-infected children have impaired endothelial function and higher levels of sCD14, a marker of immune activation, likely due to a longer cumulative duration of HIV infection. Our findings support the idea that multiple factors, including metabolic factors, inflammation and ART regimen, play a role in the progression of vascular disease and future atherosclerosis in HIV-infected children and youth. Longitudinal studies are crucial to determine the risk of cardiovascular disease in HIV-infected youths and to understand the contribution of each factors.
Acknowledgments
Funding: The work was supported by the Campbell Grant Foundation and the National Institutes of Health K23HD088295-01A1 to SDF.
The authors would like to thank the patients who participated in this research.
Source of support:
The work was supported by the Campbell Foundation and by the National Institutes of Health K23HD088295-01A1 to SDF
Sources of Funding: SDF is supported by the National Institutes of Health (K23HD088295-01A1).
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions:
SDF and GAM designed the study, SDF and GAM obtained funding. AS provided statistical support, MK and NF assisted with the inflammation and immune activation marker assays. SDF wrote the first draft of the manuscript. All authors contributed to data analysis and reviewed the manuscript for intellectual content.
Conflicts of Interest: GAM served as a consultant for Gilead, BMS, GSK/Viiv, and ICON, and has received research funding from Gilead, Merck, GSK/Viiv, and BMS. NF served as a consultant for Gilead.
References
- 1.Paula AA, Schechter M, Tuboi SH, Faulhaber JC, Luz PM, Veloso VG, et al. Continuous increase of cardiovascular diseases, diabetes, and non-HIV related cancers as causes of death in HIV-infected individuals in Brazil: an analysis of nationwide data. PLoS One. 2014;9:e94636. doi: 10.1371/journal.pone.0094636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 4.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10 -year study. J Acquir Immune Defic Syndr. 2009;51:399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terai M, Ohishi M, Ito N, Takagi T, Tatara Y, Kaibe M, et al. Comparison of arterial functional evaluations as a predictor of cardiovascular events in hypertensive patients: the Non-Invasive Atherosclerotic Evaluation in Hypertension (NOAH) study. Hypertens Res. 2008;31:1135–1145. doi: 10.1291/hypres.31.1135. [DOI] [PubMed] [Google Scholar]
- 6.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 7.Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, et al. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J. 2005;69:259–264. doi: 10.1253/circj.69.259. [DOI] [PubMed] [Google Scholar]
- 8.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 9.Poredos P. Endothelial dysfunction in the pathogenesis of atherosclerosis. Int Angiol. 2002;21:109–116. [PubMed] [Google Scholar]
- 10.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 11.Hijmering ML, Stroes ES, Pasterkamp G, Sierevogel M, Banga JD, Rabelink TJ. Variability of flow mediated dilation: consequences for clinical application. Atherosclerosis. 2001;157:369–373. doi: 10.1016/s0021-9150(00)00748-6. [DOI] [PubMed] [Google Scholar]
- 12.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 13.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Onkelinx S, Cornelissen V, Goetschalckx K, Thomaes T, Verhamme P, Vanhees L. Reproducibility of different methods to measure the endothelial function. Vasc Med. 2012;17:79–84. doi: 10.1177/1358863X12436708. [DOI] [PubMed] [Google Scholar]
- 15.Dhindsa M, Sommerlad SM, DeVan AE, Barnes JN, Sugawara J, Ley O, et al. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol (1985) 2008;105:427–432. doi: 10.1152/japplphysiol.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 17.Selamet Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, et al. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr. 2009;154:901–905. doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Mueller UM, Walther C, Adam J, Fikenzer K, Erbs S, Mende M, et al. Endothelial Function in Children and Adolescents Is Mainly Influenced by Age, Sex and Physical Activity- An Analysis of Reactive Hyperemic Peripheral Artery Tonometry. Circ J. 2017;81:717–725. doi: 10.1253/circj.CJ-16-0994. [DOI] [PubMed] [Google Scholar]
- 19.Charakida M, Donald AE, Green H, Storry C, Clapson M, Caslake M, et al. Early structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapy. Circulation. 2005;112:103–109. doi: 10.1161/CIRCULATIONAHA.104.517144. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet D, Aggoun Y, Szezepanski I, Bellal N, Blanche S. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS. 2004;18:1037–1041. doi: 10.1097/00002030-200404300-00012. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209:1156–1164. doi: 10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–595. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 25.McComsey GA, O’Riordan M, Hazen SL, El-Bejjani D, Bhatt S, Brennan ML, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. Aids. 2007;21:921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 26.Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 27.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudgins LC, Annavajjhala V, Kovanlikaya A, Frank MD, Solomon A, Parker TS, et al. Non-invasive assessment of endothelial function in children with obesity and lipid disorders. Cardiol Young. 2016;26:532–538. doi: 10.1017/S1047951115000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radtke T, Khattab K, Eser P, Kriemler S, Saner H, Wilhelm M. Puberty and microvascular function in healthy children and adolescents. J Pediatr. 2012;161:887–891. doi: 10.1016/j.jpeds.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 31.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ. Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation. 1994;90:2236–2240. doi: 10.1161/01.cir.90.5.2236. [DOI] [PubMed] [Google Scholar]
- 35.Empana JP, Canoui-Poitrine F, Luc G, Juhan-Vague I, Morange P, Arveiler D, et al. Contribution of novel biomarkers to incident stable angina and acute coronary syndrome: the PRIME Study. Eur Heart J. 2008;29:1966–1974. doi: 10.1093/eurheartj/ehn331. [DOI] [PubMed] [Google Scholar]
- 36.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansoor A, Althoff K, Gange S, Anastos K, Dehovitz J, Minkoff H, et al. Elevated NT-pro-BNP levels are associated with comorbidities among HIV-infected women. AIDS Res Hum Retroviruses. 2009;25:997–1004. doi: 10.1089/aid.2009.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duprez DA, Neuhaus J, Tracy R, Kuller LH, Deeks SG, Orkin C, et al. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS. 2011;25:651–657. doi: 10.1097/QAD.0b013e32834404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sainz T, Diaz L, Navarro ML, Rojo P, Blazquez D, Ramos JT, et al. Cardiovascular biomarkers in vertically HIV-infected children without metabolic abnormalities. Atherosclerosis. 2014;233:410–414. doi: 10.1016/j.atherosclerosis.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Ross AC, Storer N, O’Riordan MA, Dogra V, McComsey GA. Longitudinal changes in carotid intima-media thickness and cardiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J. 2010;29:634–638. doi: 10.1097/inf.0b013e3181d770c4. [DOI] [PubMed] [Google Scholar]
- 41.Steele AK, Lee EJ, Vestal B, Hecht D, Dong Z, Rapaport E, et al. Contribution of intestinal barrier damage, microbial translocation and HIV-1 infection status to an inflammaging signature. PLoS One. 2014;9:e97171. doi: 10.1371/journal.pone.0097171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nurnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schafers RF. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20:2407–2414. doi: 10.1097/00004872-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi T, Nakayama Y, Tsumura K, Yoshimaru K, Ueda H. Reflection in the arterial system and the risk of coronary heart disease. Am J Hypertens. 2002;15:405–409. doi: 10.1016/s0895-7061(02)02260-4. [DOI] [PubMed] [Google Scholar]
- 45.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]