Figure 4.
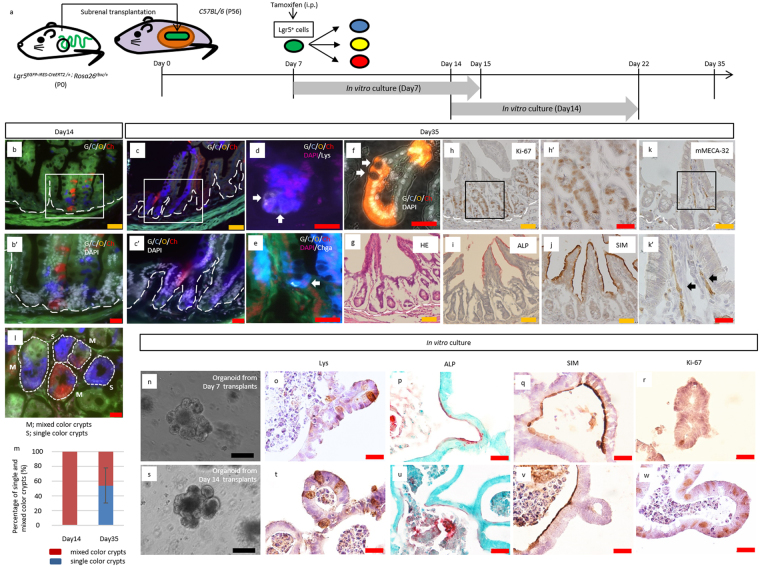
Multicolor lineage tracing of Lgr5+ cells revealed that the intestinal stem cells at P7 can autonomously differentiate into all types of cells that configure the crypt-villus unit. (a) Schematic protocol of transplanting neonatal intestine into subrenal capsule, followed by tamoxifen induction at 7 days after transplantation and harvest at 14 days or 35 days after transplantation. To investigate whether transplanted proximal regions of small intestines contained crypts with differentiation and proliferation capacities, crypts were isolated from grafts harvested at 7 or 14 days after transplantation and cultured in vitro for 8 days, at which point the resulting organoids were analyzed. P; postnatal day. (b,b’,c,c’) Fluorescent images of transplanted small intestines, in which multicolor labelling was induced in Lgr5+ cells, followed by lineage tracing until day 14 (b) or 35 (c). The number of transplants analyzed were as follows: Day 14, n = 4; Day 35, n = 8. Transplanted intestines were derived from 2 mice (D14) and 4 mice (D35), respectively. (d, e) Co-staining of the clones with the marker of differentiated lineages; Paneth cells (Lys; Lysozyme) (d), enteroendocrine cells (Chga; Chromogranin A) (e) (d,e: white arrows). (f) Representative image of Lgr5+ cell-derived clones that contained goblet cells, in which apical localization of secretory granules and compressed nuclei toward basal direction were observed (white arrows). (g) H&E staining for the transplanted small intestine harvested from P0 Lgr5 EGFP-IRES-CreERT2/+; Rosa26 rbw/+ mice at day35 after transplantation. (h,h’) Immunostaining for Ki-67 (brown), as a marker for cell proliferation, on sections of the transplanted small intestine derived from P0 Lgr5 EGFP-IRES-CreERT2/+; Rosa26 rbw/+ mice. (i) Alkaline phosphatase staining for the transplanted small intestine harvested from P0 Lgr5 EGFP-IRES-CreERT2/+; Rosa26 rbw/+ mice. (j) Immunostaining of transplanted intestinal tissue revealing maturation with the brush border cell marker sucrose-isomaltase (SIM). (k,k”) Immunostaining for mMECA-32 (brown) (k’: black arrows), as a marker of vasculature ingrowth, on sections of transplanted small intestine derived from P0 Lgr5 EGFP-IRES-CreERT2/+; Rosa26 rbw/+ mice. Higher magnification images (b’,c’,h’,k’) of the corresponding boxed area are shown below or on the right of the respective images. (l) Representative image of horizontal section of transplanted small intestine. Day 35 grafts were analyzed. M; mixed color crypts, S; single color crypts. (m) The percentage of single and mixed color crypts. Day 14, 80 crypts in 4 transplants were assessed; Day 35, 50 crypts in 8 transplants. Error bars indicate standard deviation. (n–w) Grafts were harvested at 7 (n–r) or 14 days (s–w) after transplantation and crypts were isolated from them to generate organoids for analysis at day 8. (n,s) Representative images of organoids with crypt-like structures penetrating outside. This feature rather than spherical shape demonstrated that the organoids were derived from matured crypts. (o,t) Representative images of immunostaining of organoids for Lys. Brown-stained cells indicate Lys+ Paneth cells. (p,u) Representative images of alkaline phosphatase staining of organoids. Red-stained brush border enzyme indicated that cultured organoids had expanded with differentiating into matured villi. Nuclei were stained with methyl-green. (q,v) Immunostaining of organoid revealing maturation into the cells that expressed brush border enzyme, sucrose-isomaltase (SIM). (r,w) Immunostaining for Ki-67 (brown) as a marker for cell proliferation. (o–r,t–w) Lower magnification images of the corresponding images are shown in Supplementary Fig. 13h–k, and l–o, respectively. White dashed lines indicate the boundary between epithelium and lamina propria. Scale bars: black, 100 μm; orange, 50 μm; red, 20 μm.