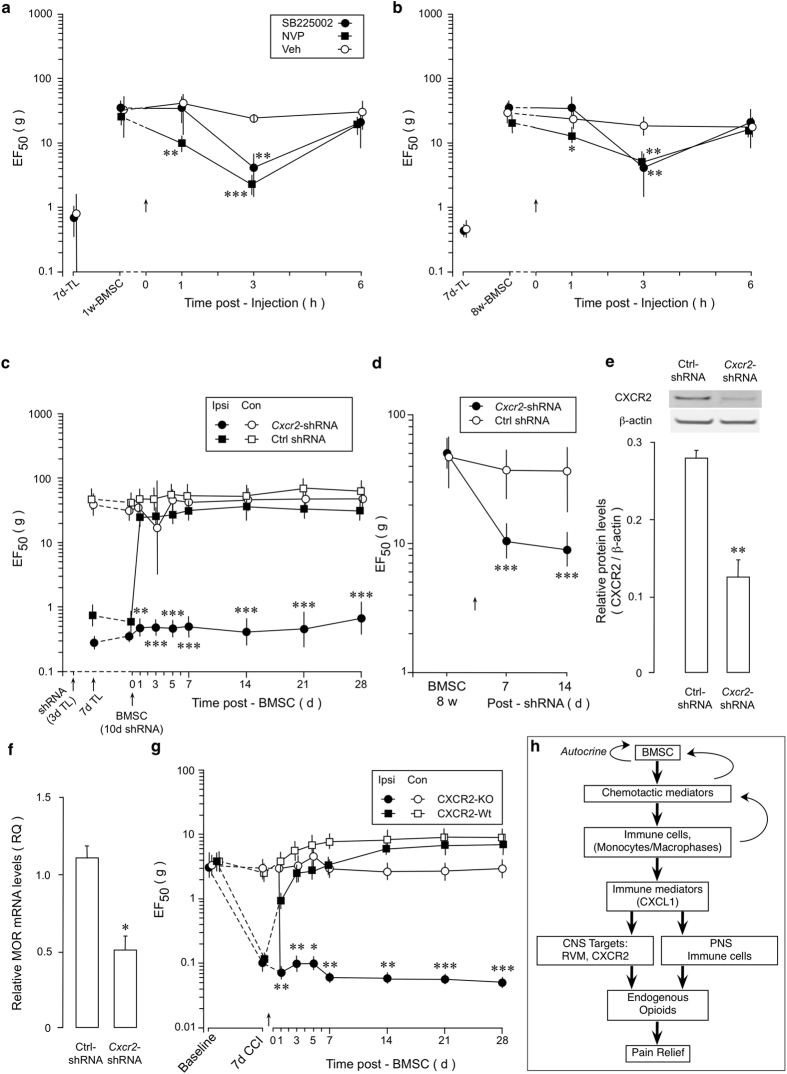
Figure 6.
Role of RVM CXCR2 in BMSC-produced antihyperalgesia. (a,b) Attenuation of BMSC-induced antihyperalgesia by a CXCR2 antagonists SB225002 and NVP CXCR2 20 (NVP) injected into the RVM at 1w (a) and 8w (b) post-BMSC injection. *-***p < 0.05-0.001, p < 0.01; vs. Veh. (c) Cxcr2 shRNA Lentivirus was microinjected into the RVM at 3d after TL and BMSCs were infused i.v. at 10d post-siRNA. Pretreatment with RNAi of Cxcr2 attenuated BMSC-produced antihyperalgesia. (d) Cxcr2 shRNA Lentivirus was microinjected into the RVM at 8w after BMSC infusion when antihyperalgesia is present. RNAi of Cxcr2 led to a reduction of EF50, or rekindle of hyperalgesia. ***p < 0.001, vs. Ctrl shRNA. (e) Western blot illustrating knock-down of CXCR2 in RVM after RNAi. Samples taken at 2w post-shRNA injection. **p < 0.01, n = 4. Cropped gel images are shown. See Supplementary Figure 9 for full-length blots. (f) RVM MOR mRNA levels were decreased in rats treated with Cxcr2 shRNA, compared to Ctrl shRNA-treated rats. Sample taken at 2w post-shRNA injection. *p < 0.05, n = 4. (g) Mice received chronic constriction injury of the infraorbital nerve (CCI-ION) to induce hyperalgesia. The antihyperalgesic effect of BMSC was absent in the CXCR2 knockout mice. *p < 0.05, **p < 0.01, ***p < 0.001, vs. CXCR2-wt. (a–d,g) Error bars are 95% confidence intervals, n = 6–8/group; (e,f) Error bars are S.E.M., n = 5/group. (h) Summary diagram of immune interactions of BMSCs in producing pain relief. Note reciprocal interactions between BMSCs and host immune cells and autocrine signaling of BMSCs (curved arrows). Immune cells may also directly release opioid peptide and contribute to pain relief through peripheral nervous system (PNS). The role of peripheral opioid in the BMSC-produced pain relief was not studied. Statistics: (a–d,g) Two-way ANOVA followed by post-hoc comparisons with Bonferroni corrections. (e,f) Unpaired, Two-tailed Student’s t-test.