Figure 4. 15.5‐kDa protein binding and induced folding of k‐turn conformation is blocked by N6‐methylation of adenine in box C/D and C’/D’ snoRNA.
-
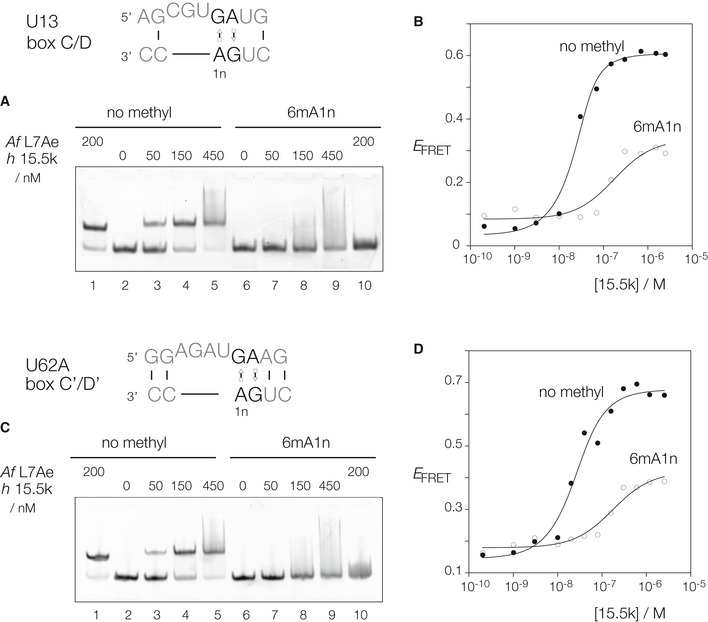
A–DTwo human snoRNA k‐turns with ‐1n = C (thus creating a GAC methylation target on the non‐bulged strand) have been chosen as examples. Human 15.5k protein binding was studied by gel electrophoretic retardation analysis (A and C) and induced folding analyzed by FRET between fluorescein and Cy3 terminally attached to a short duplex (B and D). The chosen snoRNA species were the SNORD13 (U13) box C/D (A, B) and SNORD62A (U62A) box C’/D’ (C, D) k‐turns. Each species was studied with and without N6‐methylation at the A1n position. 200 nM RNA was incubated with the indicated concentration of 15.5k, or A. fulgidus L7Ae proteins and applied to 10% polyacrylamide gels electrophoresed under non‐denaturing conditions (A and C). Binding of either protein to the unmodified RNA (tracks 1 through 5) led to the formation of discrete retarded species. At higher concentrations of 15.5k, a continuous smear of complexes ran up the gel suggesting non‐specific binding beyond stoichiometric conditions. By contrast, no specific RNA–protein complexes were observed when N6‐methyladenine‐containing RNA was used (tracks 6 through 10). FRET efficiency (E FRET) was measured as a function of 15.5k concentration for non‐methylated RNA (closed circles) and N6‐methyladenine‐containing RNA (open circles). The data have been fitted to a simple binding model (line).
