Figure EV5. Model for the functional role of the miR‐15 family at the pre‐B‐to‐immature B cell transition.
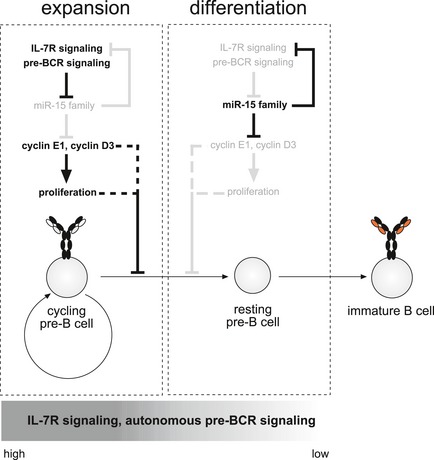
Upon entering the pre‐B cell stage, B cell precursors receive strong signals through the autonomously active pre‐BCR and are highly dependent on IL‐7 receptor signaling, which dampens the activity of the miR‐15 family. This enables strong expression of its direct and indirect targets genes such as cyclin E1 and cyclin D3, thereby promoting cell proliferation and expansion of the pre‐B cell pool. This already appears to be sufficient to counteract transcriptional reprogramming and further maturation, although we cannot exclude that this is a direct, proliferation‐independent effect of cyclin D3 and/or E1 (dotted line). However, once these cells begin to differentiate, a process that is induced by pre‐BCR signaling as well as by decreasing signals through the IL‐7R, this suppression of the miR‐15 family is reverted. Loss of pre‐BCR surface expression by downregulation of pre‐BCR components, reduced IL‐7R expression as well as decreasing IL‐7 concentrations in the microenvironment increase the activity of the miR‐15 family. This is accompanied by the miR‐15‐mediated suppression of IL‐7Ra expression, establishing an enforcing feedback loop. In consequence, the miR‐15 family becomes fully active, now being able to repress its target genes. Transcriptional downregulation of genes such as cyclin E1 and cyclin D3 further supports the transition from expansion to differentiation, ultimately resulting in the transcriptional reprogramming that is a prerequisite for proper B cell development.
