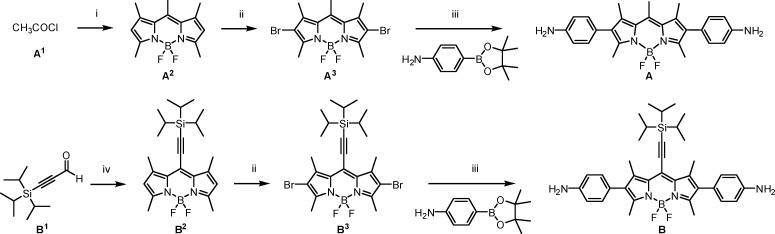
Scheme 1. Synthesis of BODIPY Ligands A and B.
Reagents and conditions: (i) (a) 2,4-dimethylpyrrole (2.0 equiv), CH2Cl2, reflux, 1 h, (b) NEt3 (3 equiv), BF3·OEt2 (4.5 equiv), CH2Cl2, rt, 10 min; (ii) NBS (3.5 equiv), CH2Cl2, rt, 1 h; (iii) Pd(dppf)Cl2·CH2Cl2 (20 mol %), K2CO3 (6.0 equiv), 1,4-dioxane/H2O (3:1), reflux (110 °C), 90 min; (iv) (a) 2,4-dimethylpyrrole (2.0 equiv), trifluoroacetic acid (5 mol %), CH2Cl2, 0 °C, 1 h, (b) DDQ (1.0 equiv), 5 min, (c) NEt3 (6.0 equiv), BF3·OEt2 (8.0 equiv), 5 min. DDQ = 2,3-dichloro-5,6-dicyano-p-benzoquinone, NBS = N-bromosuccinimide, dppf = 1,1′-bis(diphenylphosphino)ferrocene.