Abstract
Axial spondyloarthritis (SpA) is a spectrum of inflammatory disease with stages characterized by both nonradiographic and radiographic sacroiliitis. Nonradiographic axial SpA is associated with health-related quality-of-life impairment and may progress to ankylosing spondylitis. Axial SpA has a low prevalence in some countries in North Africa and the Middle East, and pooling of data and resources is needed to increase understanding of the regional picture. Early diagnosis and effective treatment are required to reduce disease burden and prevent progression. Anti-TNF therapy is recommended for patients with persistently high disease activity despite conventional treatment, and has been shown to be effective in patients without radiographic damage. Diagnostic delays can be an obstacle to early treatment and appropriate referral strategies are needed. In some countries, restricted access to magnetic resonance imaging and anti-TNF agents presents a challenge. In this article, a group of experts from North Africa and the Middle East evaluated the diagnosis and management of axial SpA with particular reference to this region.
Keywords: North Africa and Middle East, ankylosing spondylitis, axial spondyloarthritis, inflammatory back pain, sacroiliitis, TNF inhibitors
Introduction
Axial spondyloarthritis (SpA) is a spectrum of inflammatory disease with stages characterized by both nonradiographic and radiographic sacroiliitis.1 Sacroiliac joint involvement is considered to be the hallmark of SpA, and the disease course is characterized by ongoing axial inflammation and radiographic progression, associated with restricted mobility of the spine and decreased function.2 The Assessment of Spondyloarthritis International Society (ASAS) classification criteria define axial SpA as either the presence of sacroiliitis by radiography or by magnetic resonance imaging (MRI) plus at least one SpA feature (“imaging arm”), or the presence of human leukocyte antigen (HLA)-B27 plus at least two SpA features (“clinical arm”).3 This diagnostic method is more reliable than older criteria (ESSG4 or Amor5), which were developed before MRI was widely used. In addition, the ASAS classification criteria enable early diagnosis and treatment of axial SpA,6 reducing signs and symptoms and decreasing the risk of radiographic progression and further functional impairment.7
Patients with nonradiographic axial SpA are demographically similar to those with radiographic disease (ankylosing spondylitis [AS]).2,8 Women are more likely than men to have nonradiographic disease, while men are more likely than women to have radiographic forms, and patients with AS are more likely to have a family history of SpA compared with those with nonradiographic disease.2,8 Both groups are similar in terms of comorbidities, clinical characteristics, disease activity index (Bath Ankylosing Spondylitis Disease Activity Index; BASDAI), and the proportion of patients treated with nonsteroidal anti-inflammatory drugs (NSAIDs). Patients with AS tend to have higher C-reactive protein (CRP) levels, and worse function (Bath Ankylosing Spondylitis Functional Index; BASFI) and spinal mobility (Bath Ankylosing Spondylitis metrology index; BASMI) than those with nonradiographic disease. By definition, patients with AS have radiographic sacroiliitis, whereas those with nonradiographic axial SpA have a lower modified Stoke Ankylosing Spondylitis Spine Score (mSASSS).2,8 Spinal inflammation, as assessed by MRI, is seen in 60% of patients with AS and 47% of those with nonradiographic axial SpA.2
Nonradiographic axial SpA is a subset of axial SpA in which no clear structural damage is visible using conventional radiography. The term includes patients with early radiographic sacroiliitis (grade 1 bilateral or grade 2 unilateral) as well as those with none. While some patients will progress to AS over time, others may never develop radiographic sacroiliitis, but may have a high burden of disease.7 The rate of progression of nonradiographic axial SpA to AS appears to be ∼10% over 2 years, with a higher rate (around 20%) in patients with elevated CRP levels or active inflammation of sacroiliac joints on MRI.9
This article will discuss the prevalence, diagnosis and management of axial SpA (both radiographic and nonradiographic), with particular reference to the Africa and Middle East region, and will consider the associated educational needs. A group of Africa and Middle East regional experts discussed key issues relating to the disease and its management, then completed an in-depth questionnaire on the subject. Feedback from these resources is cited where relevant to gain an insight into the challenges presented by axial SpA in North Africa and the Middle East. As a result of the lack of published information about SpA in the region, particularly in Africa, much of this article is based on expert opinion.
Prevalence of nonradiographic axial SpA
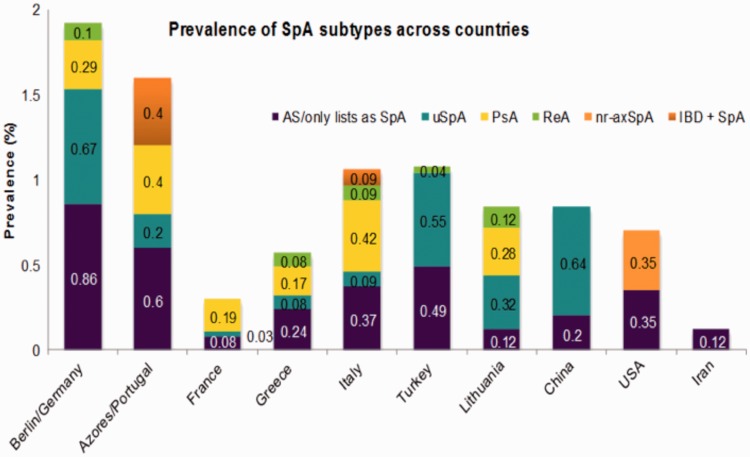
Data regarding the prevalence of SpA in various countries are shown in Figure 1.10–19 The age-adjusted prevalence of SpA in the USA is estimated to be 0.9% (Amor criteria) or 1.4% (ESSG criteria), with no significant sex differences.10 When using the ASAS criteria, the US prevalence was 0.7%, with estimates of 0.35% each for AS and nonradiographic axial SpA.20 Studies in Europe have estimated the annual incidence rate of SpA to be 19–52 per 100 000 people, compared with 24–36 for rheumatoid arthritis (RA).21 SpA seems to be more prevalent than RA in the majority of, but not all, European populations.21 A study in a Chinese Han population found a prevalence of axial SpA of 0.78%,11 while a literature review reported pooled prevalences of 0.24% and 0.23% for AS, and 0.45% and 0.93% for undifferentiated SpA in China.11 The prevalence of spondyloarthropathies in Japan is thought to be lower than in Caucasian populations.12,21 In Pakistan, the prevalence of SpA was reported to be 0.3%.22 Estimates of the proportion of nonradiographic cases in patients with axial SpA vary between 20% and 80%.9
Figure 1.
Prevalence of axial spondyloarthritis (SpA) subtypes in various countries. Data extracted from published studies.10–19 uSpA, undifferentiated spondyloarthritis; PsA, psoriatic arthritis; ReA, reactive arthritis; nr-axSpA, nonradiographic axial spondyloarthritis; IBD + SpA, inflammatory bowel disease with spondyloarthritis.
Data for the North Africa and Middle East region are scarce. An Iranian study reported the prevalence of SpA, AS and RA to be 0.23%, 0.12% and 0.33%, respectively.23 A study of 2500 people in Kuwait found only one patient with AS21 and a study in Saudi Arabia found no cases of AS.24 Another Saudi Arabian group reviewed the medical charts of people diagnosed with AS between 1988 and 1991 at the King Khalid University Hospital and identified only 15 cases.25 An investigation performed in one of the three general hospitals in Abu Dhabi reviewed the medical records of the 28 residents diagnosed with AS between 1987 and 1996. Of these, 11 patients were Asian and none of the 17 Arabs came from the local community.25 A study of 518 patients with SpA (97% with sacroiliitis) in Morocco, Algeria and Tunisia reported a high rate of hip involvement (37%).26 AS is exceptionally rare among sub-Saharan Africans.21
The prevalence of HLA-B27 positivity among Arabs in the United Arab Emirates is extremely low (0.5%) compared with other populations (e.g. UK, 8%; Haida [First Nations, Canada], 50%27),28 suggesting a possible explanation for the low prevalence of AS in this population.29 HLA-B27 positivity is a requirement for classification of axial SpA in the clinical arm of the ASAS criteria,3 and is strongly associated with AS.30 It appears to be associated with a worse prognosis of axial SpA and to be less common in patients without radiographic sacroiliitis.31 In general, there is a close correlation between HLA-B27 prevalence and AS prevalence in the worldwide population,32 but this association may be weaker in Arab countries than in European populations. Approximately 2–5% of individuals in the major Arab populations are positive for HLA-B27, rising to around 70% in patients with AS. In contrast, in northern Europe, ∼90% of patients with AS are positive for HLA-B27.32 Evidence also suggests phenotypic differences between HLA-B27-positive and HLA-B27-negative patients with AS.33
There is a clear need for further information on the prevalence of SpA in general, as well as nonradiographic axial SpA in the Africa and Middle East region. Studies are underway to gather more data from local hospitals, and registries should be established in each country. These studies and registries should pool resources in order to provide a more extensive understanding of the regional picture.
Diagnosis of nonradiographic axial SpA: Challenges within the Africa and Middle East region
Delay between symptom onset and diagnosis
The burden of disease due to axial SpA is high. Levels of general pain, pain at night, fatigue and health-related quality-of-life impairment are equally high in both AS and nonradiographic axial SpA.2,8 A study in the UK reported substantial productivity losses associated with AS,34 and a multicentre study of the tumour necrosis factor (TNF) inhibitor, certolizumab pegol, found a similarly high burden on workplace and household productivity at baseline in patients with AS and those with nonradiographic axial SpA.35 Early diagnosis and effective treatment are needed to reduce this burden.
Follow-up of a German cohort of patients with early axial SpA showed that 20% of those with AS and 7% of those with nonradiographic axial SpA had experienced radiographic progression (a worsening in mSASSS of ≥2 over 2 years). Of those with nonradiographic axial SpA at baseline, 12% had progressed to definite radiographic sacroiliitis.36 The presence of syndesmophytes at baseline, elevated systemic inflammation markers (erythrocyte sedimentation rate [ESR] or CRP) and cigarette smoking were predictive of progression.
A major obstacle to early treatment of SpA is the 5–10 year delay between the appearance of the first (chronic) symptoms of the disease and diagnosis.37 A German study reported an average delay between first SpA symptoms and AS diagnosis of 8.3 years for HLA-B27-positive patients and 11.4 years for HLA-B27-negative patients.33 The longer delay in HLA-B27-negative patients may have implications in North Africa and the Middle East. A study in four countries in the North Africa and Middle East region found an average delay from onset of symptoms to AS diagnosis of 4.9 years.38 One reason for the delay in diagnosis is the low awareness of AS and axial SpA in general among nonrheumatologists. In the past, delays were related to the relatively late appearance of radiographic sacroiliitis and the requirement for bilateral grade 2 or unilateral grade 3 or 4 sacroiliitis before a diagnosis of AS could be made.37 The ASAS criteria now allow a diagnosis of axial SpA to be made in the absence of such radiographic evidence.3
The regional experts from North Africa and the Middle East reported average times from symptom onset to diagnosis of axial SpA in the range of 3–10 years, with one respondent stating 6–12 months. The time delays are similar to those in other regions.39 Delays for nonradiographic axial SpA covered a similar range, with several respondents citing a slightly longer delay for this disease type. The regional experts believe that this may be due to reasons including the fact that low back pain is common in the general population, and an inflammatory origin of back pain is not as carefully sought by primary care physicians and orthopaedic specialists (who may be the first to encounter such patients). There is also a general lack of pathognomonic clinical features or laboratory tests for AS, with ESR and CRP concentrations raised in only 50–70% of patients with active disease.
Diagnostic criteria
A German study evaluated two screening strategies for early identification of patients with axial SpA in primary care.39 In one, patients with chronic back pain (>3 months) were referred to rheumatology if they had inflammatory back pain, were HLA-B27 positive, or had sacroiliitis detected by imaging. In the other strategy, such patients were referred if they had two of five criteria comprising the three listed above plus a family history of AS or a good treatment response to NSAIDs. For both strategies, ∼40% of patients identified were diagnosed by the rheumatologist as having definite axial SpA, demonstrating their potential as easy and reliable screening methods for axial SpA in primary care.39 However, cultural and genetic differences seen in North Africa and the Middle East are likely to affect screening strategies, and any such strategies would need to be validated or developed locally.
It must be remembered that the identification of features of the ASAS criteria is not sufficient to make a diagnosis. Clinicians need to use “pattern recognition”, exclude other common conditions first, and explicitly consider the pros and cons of a specific potential diagnosis.7 In Germany, patients with chronic low back pain who were referred to rheumatology were stratified according to four criteria: morning stiffness >30 min; improvement on movement but not on rest; waking up in the second half of the night because of back pain; improvement with NSAIDs within 48 h.6 No single criterion was predictive of a diagnosis of axial SpA, but at least three items demonstrated good sensitivity and specificity.6 This shows that preselecting primary care patients with back pain based on a combination of clinical items is diagnostically useful in axial SpA. This type of approach may be viable in North Africa and the Middle East.
The typical profile of a patient with nonradiographic axial SpA in the North Africa and Middle East region is a young patient with inflammatory lower back pain, poor response to analgesia, normal X-radiography examination, asymmetrical arthritis and unexplained ankle swelling with Achilles tendinitis. The majority of experts practising in this region use the ASAS classification criteria to diagnose nonradiographic axial SpA; as in other regions, there remain key challenges in their use for diagnosis. Establishing and confirming an early diagnosis is one of the most pressing issues for those physicians diagnosing nonradiographic axial SpA, and may be a reason for the slightly longer delay in diagnosis of the nonradiographic disease, highlighted above. As discussed previously, a low proportion of patients in the region are HLA-B27-positive.28 Therefore, using the ASAS criteria may lead to patients only being identified in the imaging arm of the criteria, and if these are used as diagnostic criteria this may limit and further delay diagnosis within the region.
Role of imaging in axial SpA diagnosis
The imaging arm of the ASAS criteria includes active (acute) inflammation on MRI that is highly suggestive of sacroiliitis associated with SpA.3 An ASAS/OMERACT working group defined a positive spinal MRI for inflammation as the presence of anterior/posterior spondylitis in at least three sites. Evidence of fatty deposition at several vertebral corners was found to be suggestive of axial SpA, especially in younger adults.40
In the North Africa and Middle East region, MRI is widely available in hospitals and there is a good level of understanding of its value among physicians, but there are issues restricting its use. There may be a long waiting list for MRI in some of the hospitals in the region, which may be another reason for the delay in diagnosis. Due to the healthcare systems in the region and the costs associated with MRI, insurance companies may also be unwilling to fund its use, thus restricting access for certain patient groups. There is also difficulty in identifying the changes of sacroiliitis by general physicians, orthopaedic specialists and even sometimes by radiologists.
Measures of disease activity
Disease measures used in the diagnosis and management of patients in the North Africa and Middle East region include the differing physical components of the disease, such as morning stiffness (duration of spinal stiffness in the past week), lumbar flexion (Schober’s test), chest expansion, lateral spinal flexion, occiput-to-wall distance and tragus-to-wall distance. Laboratory measures such as ESR and CRP are also utilized. The regional experts from North Africa and the Middle East reported that traditional scoring methods including BASDAI (described as being simple to use), BASFI, Ankylosing Spondylitis Disease Activity Score (ASDAS), BASMI and visual-analogue scales to evaluate spinal pain, and ASQoL to measure health-related quality of life, are regularly used for patient visits.41
Further educational needs
When asked what further education is needed to help with the understanding of inflammatory back pain, how to recognize it and hence how to diagnose nonradiographic disease, the regional experts identified several issues. The authors feel that there is a need to teach general physicians, internal medicine specialists, physiotherapists and orthopaedic surgeons about inflammatory back pain and how to recognize it early. This should reduce the time from the onset of symptoms to referral to a rheumatologist and eventual diagnosis. Improved knowledge of referral criteria is also needed to expedite the process. Educational resources could be created through the development of case studies, allowing healthcare professionals in the region to follow a case and discuss the measures taken.
There is also a need for continuing medical education for rheumatologists to improve the knowledge in the field, particularly regarding imaging procedures. The interpretation of conventional radiographs and MRI can be difficult and hence further training on this is required within the region.
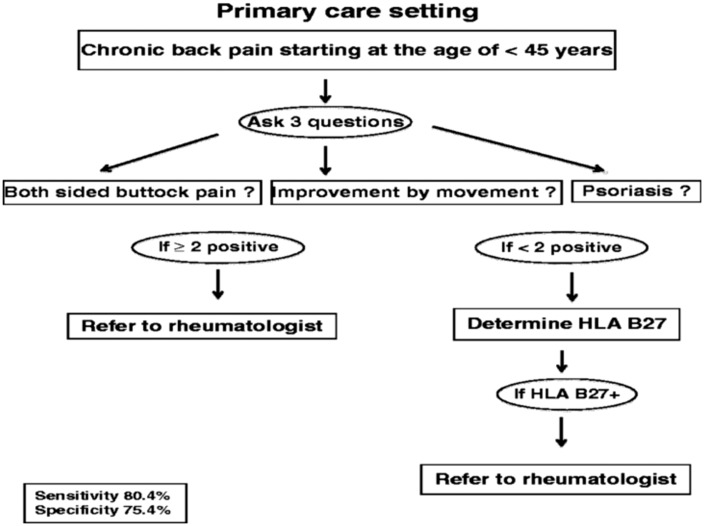
Referral strategies tailored to the situation in North Africa and the Middle East are needed. In Germany, a two-step strategy combining three clinical questions with HLA-B27 performed well in the identification of patients with axial SpA in primary care (Figure 2).42 Using this approach, determination of HLA-B27 was needed in only half of the patients suspected of having axial SpA, which may be relevant for the Africa and Middle East situation where HLA-B27 positivity is low.
Figure 2.
Two-phase strategy for identifying axial spondyloarthritis in the primary care setting.41 HLA, human leukocyte antigen.
At a basic level, there is also a need to further understand the terminology of the spectrum of disease and to ensure that this in keeping with the latest developments in the field.
Management of nonradiographic axial SpA: Challenges within the Africa and Middle East region
Recommendations for treatment
The ASAS/EULAR recommendations for the management of axial SpA state that treatment should be tailored according to the current manifestations of disease, the level of current symptoms, clinical findings and prognostic indicators, and the general clinical status (e.g. comorbidities and concurrent medications).43 NSAIDs are the recommended first line treatments, and continuous therapy may be needed in those patients with persistent active disease. There is no evidence for the efficacy of systemic glucocorticoids or disease-modifying antirheumatic drugs such as sulphasalazine or methotrexate in axial disease. Anti-TNF therapy should be given to patients with persistently high disease activity despite receiving conventional treatments.40
TNF inhibitors
The efficacy of TNF inhibitors in patients with AS is well established.43,44 Several clinical trials have been carried out to assess the efficacy of TNF inhibitors in nonradiographic axial SpA. A group of 215 patients with nonradiographic axial SpA were randomized to receive 50 mg/week etanercept or placebo for a 12-week double-blind treatment phase followed by an open-label period of etanercept treatment.45 At baseline, 72% of patients were HLA-B27 positive and 81% were MRI sacroiliitis positive. Etanercept significantly reduced MRI sacroiliac joint and spinal inflammation scores versus placebo at week 12 (–46.9% vs −10.9%; P < 0.001, and −45.4% vs −33.4%; P = 0.041, respectively); 33% of etanercept-treated patients and 15% of placebo patients achieved ASAS40 by 12 weeks. Post-hoc analyses suggested a possible association between higher baseline CRP levels or sacroiliac joint MRI inflammation scores and higher ASAS40 response rates to etanercept. At week 24, patients who switched to etanercept from placebo at 12 weeks achieved improvement similar to those receiving etanercept for 24 weeks.45 By week 48, 53% of patients in the combined groups (etanercept for 12 weeks, then continued for 36 weeks and placebo for 12 weeks followed by 36 weeks of etanercept treatment) had achieved ASAS40.46
The ESTHER trial randomized 76 patients with early axial SpA to treatment with 25 mg etanercept twice weekly or 2–3 mg/day sulphasalazine. Whole-body MRI showed significantly greater reductions in sacroiliac joint score and spine inflammation after 48 weeks in patients treated with etanercept, compared with those on sulphasalazine.47,48 ASAS plus MRI remission at week 48 was reached significantly more often in patients treated with etanercept than with sulphasalazine (33% vs 11%; P = 0.03).47,48 Post-hoc analysis of patients with AS (n = 20) or nonradiographic axial SpA (n = 20) treated with etanercept showed a similarly good response at 48 weeks in terms of BASDAI, ASDAS and MRI scores in the two groups.49 After 3 years’ continuous etanercept treatment, there was a consistently effective suppression of osteitis on MRI in patients with early axial SpA and a very low rate of new onset of osteitis.50
In the ABILITY-1 study, patients with nonradiographic axial SpA (n = 185) were randomized to receive 40 mg adalimumab every other week or placebo. Significantly more patients in the adalimumab group achieved ASAS40 at week 12 compared with patients in the placebo group (36% vs 15%; P < 0.001); improvements in ASDAS, BASDAI and health-related quality-of-life measures were also seen with adalimumab. Inflammation in the spine and sacroiliac joints on MRI significantly decreased after 12 weeks’ adalimumab treatment.51 Most adalimumab-treated patients who were in clinical remission after up to 2 years’ therapy also had MRI remission; however, resolution or absence of inflammation on MRI did not always correspond to clinical remission.52
In the RAPID-axSpA trial, patients with active SpA (n = 325) were randomized to receive placebo or 200 mg certolizumab pegol every 2 weeks or 400 mg every 4 weeks. At week 12, ASAS20 responses were higher in the certolizumab pegol treatment arms than in the placebo group (58%, 64% and 38%, respectively; P ≤ 0.004). At week 24, the combined active treatment arms showed significant changes from baseline versus placebo for BASFI, BASDAI and BASMI. Similar improvements were reported with certolizumab pegol versus placebo in both patients with AS or nonradiographic axial SpA.53 Improvements in SPARCC MRI sacroiliac joint scores and ASspiMRI-a Berlin modification were observed at 12 weeks in both certolizumab pegol arms compared with placebo in the overall population and both subpopulations.54 The improvements in clinical efficacy and patient-reported outcomes at 24 weeks were sustained to 96 weeks in open-label treatment, with similar sustained improvements in both AS and nonradiographic axial SpA subpopulations.55The GO-AHEAD study randomized 198 patients with active nonradiographic axial SpA to placebo or 50 mg golimumab every 4 weeks. The primary endpoint, ASAS20 response at week 16, was achieved by significantly more patients in the golimumab group than in the placebo group (71% vs 40%; P < 0.0001). Significantly more golimumab-treated than placebo-treated patients also achieved ASAS40 response, BASDAI 50 response, ASAS partial remission and change from baseline in SPARCC MRI sacroiliac joint scores.56
Four TNF inhibitors are licensed in Europe for the treatment of nonradiographic axial SpA, in addition to AS. Etanercept is indicated for treatment of adults with severe nonradiographic axial SpA with objective signs of inflammation (as indicated by elevated CRP levels and/or MRI evidence), who have had an inadequate response to NSAIDs.57 Adalimumab, certolizumab pegol and golimumab are indicated in adults with severe active axial SpA without radiographic evidence of AS but with objective signs of inflammation (elevated CRP levels and/or positive MRI), who have had an inadequate response to, or are intolerant to NSAIDs.58–60 Although etanercept, adalimumab, certolizumab pegol and golimumab are not licensed for use in nonradiographic axial SpA in the North Africa and Middle East region, these therapies are utilized, as discussed below.
Management in the Africa and Middle East region
The regional experts tend to use the EULAR/ASAS/international guidelines for treating axial SpA. For example, one respondent would usually start NSAIDs for 2–3 weeks and then add a TNF inhibitor if two NSAIDs failed. Another would use a TNF inhibitor if treatment with at least two NSAIDs failed over 1 month (BASDAI ≥ 4 or ASDAS ≥ 2.1). The challenges cited included the cost of TNF inhibitor drugs; however, this is dependent on reimbursement within the healthcare system and, for insured patients, the insurance companies’ willingness to pay. Some physicians have encountered problems in persuading patients to take the drugs. This may be due to the chronic nature of the disease and young patients’ fear of being on long-term therapy. Retention rates on TNF inhibitors are, therefore, important to both patient and physician. In Europe, registry data of patients with AS indicates a significantly longer drug survival time for etanercept than monoclonal antibody TNF inhibitors.44 Secondary failure impacts on drug survival, and one reason for this is immunogenicity. Although TNF inhibitor monoclonal antibodies have been shown to lead to the development of neutralizing antibodies that impact efficacy, this is not the case for etanercept.61 In real-world data, patients who were antidrug antibody-positive continued therapy for less time than those who were antidrug antibody-negative.62 Another explanation for the reluctance of patients is the method of administration. Infliximab is administered by infusion and hence requires time in hospital, but the other TNF inhibitors can be administered subcutaneously.57–60
Further measures are required before initiating TNF inhibitor therapy. Latent tuberculosis (TB) is an issue in the Africa and Middle East region, with positive TB tests occurring in ∼20–25% of patients in the experts’ clinics, in accordance with published data.63 The hospital of the lead authors, for example, is a university tertiary care centre where the Rheumatology unit is staffed by eight full-time rheumatologists. Each year, 14000–16000 patients are referred to this clinic from primary health centres, secondary care hospitals and private hospitals. The population in the Gulf area is a mix of nationals, and a large number of expatriates from Asia and Africa. TB testing should always be carried out before therapy is initiated; patients are retested once a year in some, but not all, of the countries in the region. Long-term and registry data have demonstrated a low rate of serious infections, including TB, in patients with SpA receiving etanercept.64,65 The risk of contracting TB is lower with etanercept than with TNF inhibitor monoclonal antibodies. Hepatitis testing is also carried out before therapy is started in the majority of countries, and the incidence of hepatitis B virus (HBV) infection is ∼3–4% in the experts’ clinics. Before initiating TNF inhibitor treatment, it is also recommended to screen patients for both HBV and hepatitis C virus (HCV) infection (based on anti-HCV antibodies). In patients who are HBV negative, vaccination is recommended before initiation of anti-TNF therapy. In addition, for some patients it is advisable to monitor liver enzymes and test for HCV regularly every 3 months. In patients with a history of HBV, monitoring of liver enzymes, HBsAg, and HBV DNA every month and then every 3 months is recommended. Management of SpA with viral hepatitis is a great challenge and needs close collaboration with the gastroenterologist for management of these patients with antiviral and anti-TNF therapy.65
HIV may play a role in the pathogenesis of certain types of SpA in sub-Saharan Africa,66 and is a consideration for treatment in the North Africa and Middle East region. Data on this and other infections in the region are lacking, and the establishment of regional registries would be valuable in the collection of such data.
Other considerations include the fact that axial SpA is an independent cardiovascular risk factor; chronic inflammation contributes to the increased atherosclerosis risk in patients with axial SpA.67,68 This may be a contraindication for NSAIDs as well as selective cyclo-oxygenase inhibitors. There are several other safety aspects related to NSAID treatment, such as gastrointestinal, renal, hepatic and allergic reactions. Cancers, infections and osteoporosis could, in part, explain the increased mortality observed in patients with axial SpA.69
Other comorbidities that should be considered in patients with nonradiographic axial SpA include diabetes and hypertension. There is a need for good communication between rheumatologists and other specialties in the region, in order to provide treatment for the patients that is effective and treats each aspect of their clinical conditions.
Conclusions
Nonradiographic axial SpA is a subset of axial SpA with no clear structural damage as defined by conventional radiography, which is associated with health-related quality-of-life impairment and may progress to AS.2,8,36 Early diagnosis and effective treatment are needed to reduce this burden, but diagnostic delay can be a major obstacle.37 The authors of this article report average times from symptom onset to diagnosis of axial SpA in Africa and the Middle East that are similar to those in other regions. Longer delays for the diagnosis of nonradiographic SpA may be related to lack of knowledge about inflammatory back pain among primary care physicians and orthopaedic specialists, as well as to lack of access to MRI among some patient groups. Continuing medical education and regionally appropriate referral strategies are needed to improve this situation. International recommendations state that anti-TNF therapy should be given to patients with axial SpA with persistently high disease activity, despite conventional treatments.43 Access to these agents may present a challenge in some countries in the region, and persuading some patients to start long-term therapy may be difficult. Other considerations include the need for testing for TB, HBV and HCV. The establishment of registries of patients with SpA in each country within the region would be an important step in ensuring the best possible management of these patients in future.
Acknowledgement
Initial drafting and subsequent medical writing support was provided by Clare Griffith, Synergy, London, UK, and funded by Pfizer.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This paper was based on proceedings of an advisory board that was funded by Pfizer. K.S. is an employee of Pfizer. M.H. has received honoraria as speaker from Abbvie, Pfizer, Roche and Jansen, and for participation in international drug company sponsored research trials from Roche, Abbvie and Pfizer.
References
- 1.Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005; 52: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 2.Kiltz U, Baraliakos X, Karakostas P, et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012; 64: 1415–1422. [DOI] [PubMed] [Google Scholar]
- 3.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 4.Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991; 34: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 5.Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic 1990; 57: 85–89. [PubMed] [Google Scholar]
- 6.Braun A, Saracbasi E, Grifka J, et al. Identifying patients with axial spondyloarthritis in primary care: how useful are items indicative of inflammatory back pain? Ann Rheum Dis 2011; 70: 1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deodhar A, Reveille JD, van den Bosch F, et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the assessment of spondyloarthritis international society in response to the US food and drug administration’s comments and concerns. Arthritis Rheumatol 2014; 66: 2649–2656. [DOI] [PubMed] [Google Scholar]
- 8.Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German spondyloarthritis inception cohort. Arthritis Rheum 2009; 60: 717–727. [DOI] [PubMed] [Google Scholar]
- 9.Sieper J, van der Heijde D. Review: nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum 2013; 65: 543–551. [DOI] [PubMed] [Google Scholar]
- 10.Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res (Hoboken) 2012; 64: 905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao ZT, Pan YF, Huang JL, et al. An epidemiological survey of low back pain and axial spondyloarthritis in a Chinese Han population. Scand J Rheumatol 2009; 38: 455–459. [DOI] [PubMed] [Google Scholar]
- 12.Hukuda S, Minami M, Saito T, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan ankylosing spondylitis society. J Rheumatol 2001; 28: 554–559. [PubMed] [Google Scholar]
- 13.Trontzas P, Andrianakos A, Miyakis S, et al. Seronegative spondyloarthropathies in Greece: a population-based study of prevalence, clinical pattern, and management. The ESORDIG study. Clin Rheumatol 2005; 24: 583–589. [DOI] [PubMed] [Google Scholar]
- 14.Onen F, Akar S, Birlik M, et al. Prevalence of ankylosing spondylitis and related spondyloarthritides in an urban area of Izmir, Turkey. J Rheumatol 2008; 35: 305–309. [PubMed] [Google Scholar]
- 15.Adomaviciute D, Pileckyte M, Baranauskaite A, et al. Prevalence survey of rheumatoid arthritis and spondyloarthropathy in Lithuania. Scand J Rheumatol 2008; 37: 113–119. [DOI] [PubMed] [Google Scholar]
- 16.Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41: 58–67. [DOI] [PubMed] [Google Scholar]
- 17.Bruges-Armas J, Lima C, Peixoto MJ, et al. Prevalence of spondyloarthritis in Terceira, Azores: a population based study. Ann Rheum Dis 2002; 61: 551–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Angelis R, Salaffi F, Grassi W. Prevalence of spondyloarthropathies in an Italian population sample: a regional community-based study. Scand J Rheumatol 2007; 36: 14–21. [DOI] [PubMed] [Google Scholar]
- 19.Saraux A, Guillemin F, Guggenbuhl P, et al. Prevalence of spondyloarthropathies in France: 2001. Ann Rheum Dis 2005; 64: 1431–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strand V, Rao SA, Shillington AC, et al. Prevalence of axial spondyloarthritis in United States rheumatology practices: assessment of spondyloarthritis international society criteria versus rheumatology expert clinical diagnosis. Arthritis Care Res (Hoboken) 2013; 65: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 21.Akkoc N. Are spondyloarthropathies as common as rheumatoid arthritis worldwide? a review. Curr Rheumatol Rep 2008; 10: 371–378. [DOI] [PubMed] [Google Scholar]
- 22.Farooqi A, Gibson T. Prevalence of the major rheumatic disorders in the adult population of north Pakistan. Br J Rheumatol 1998; 37: 491–495. [DOI] [PubMed] [Google Scholar]
- 23.Davatchi F, Jamshidi AR, Banihashemi AT, et al. WHO-ILAR COPCORD study (stage 1, urban study) in Iran. J Rheumatol 2008; 35: 1384–1384. [PubMed] [Google Scholar]
- 24.Rajapakse CN. The spectrum of rheumatic diseases in Saudi Arabia. Br J Rheumatol 1987; 26: 22–23. [DOI] [PubMed] [Google Scholar]
- 25.al-Arfaj A. Profile of ankylosing spondylitis in Saudi Arabia. Clin Rheumatol 1996; 15: 287–289. [DOI] [PubMed] [Google Scholar]
- 26.Claudepierre P, Gueguen A, Ladjouze A. Predictive factors of severity of spondyloarthropathy in North Africa. Br J Rheumatol 1995; 34: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan NJ. The ramifications of HLA-B27. J R Soc Med 2004; 97: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.al-Attia HM, al-Amin N. HLA-B27 in healthy adults in UAE: an extremely low prevalence in Emirian Arabs. Scand J Rheumatol 1995; 24: 225–227. [DOI] [PubMed] [Google Scholar]
- 29.al Attia HM, Sherif AM, Hossain MM, et al. The demographic and clinical spectrum of Arab versus Asian patients with ankylosing spondylitis in the UAE. Rheumatol Int 1998; 17: 193–196. [DOI] [PubMed] [Google Scholar]
- 30.Brewerton DA, Hart FD, Nicholls A, et al. Ankylosing spondylitis and HL-A 27. Lancet 1973; 301: 904–907. [DOI] [PubMed] [Google Scholar]
- 31.Chung HY, Machado P, van der Heijde D, et al. HLA-827 positive patients differ from HLA-827 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis 2011; 70: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa KN, Hammoudeh M, Khan MA. HLA-B27 prevalence in Arab populations and among patients with ankylosing spondylitis. J Rheumatol 2012; 39: 1675–1677. [DOI] [PubMed] [Google Scholar]
- 33.Feldtkeller E, Khan MA, van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. Positive patients with ankylosing spondylitis. Rheumatol Int 2003; 23: 61–66. [DOI] [PubMed] [Google Scholar]
- 34.Rafia R, Ara R, Packham J, et al. Healthcare costs and productivity losses directly attributable to ankylosing spondylitis. Clin Exp Rheumatol 2012; 30: 246–253. [PubMed] [Google Scholar]
- 35.Van der Heijde D, Braun J, Rudwaleit M, et al. Reduction of disease burden on workplace and household productivity in axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis, over 48 weeks of treatment with certolizumab pegol. Presented at ACR 2013, abstract 1520.
- 36.Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012; 64: 1388–1398. [DOI] [PubMed] [Google Scholar]
- 37.Sieper J, Rudwaleit M. Early referral recommendations for ankylosing spondylitis (including pre-radiographic and radiographic forms) in primary care. Ann Rheum Dis 2005; 64: 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hammoudeh M, Al Rayes H, Alawadhi A, et al. Spondyloarthritides in the Middle East and the challenges in their management. Ann Rheum Dis 2015; 74: 1160–1161.
- 39.Poddubnyy D, Vahldiek J, Spiller I, et al. Evaluation of 2 screening strategies for early identification of patients with axial spondyloarthritis in primary care. J Rheumatol 2011; 38: 2452–2460. [DOI] [PubMed] [Google Scholar]
- 40.Hermann KG, Baraliakos X, van der Heijde DM, et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis 2012; 71: 1278–1288. [DOI] [PubMed] [Google Scholar]
- 41.Zochling J. Measures of Symptoms and Disease Status in Ankylosing Spondylitis. Arthritis Care Res (Hoboken) 2011; 63(Suppl 11): S47–S58. [DOI] [PubMed] [Google Scholar]
- 42.Braun A, Gnann H, Saracbasi E, et al. Optimizing the identification of patients with axial spondyloarthritis in primary care–the case for a two-step strategy combining the most relevant clinical items with HLA B27. Rheumatology (Oxford) 2013; 52: 1418–1428. [DOI] [PubMed] [Google Scholar]
- 43.Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011; 70: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavelka K, Forejtová S, Stolfa J, et al. Anti-TNF therapy of ankylosing spondylitis in clinical practice. Results from the Czech national registry ATTRA. Clin Exp Rheumatol 2009; 27: 958–963. [PubMed] [Google Scholar]
- 45.Dougados M, van der Heijde D, Sieper J, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2014; 66: 2091–2102. [DOI] [PubMed] [Google Scholar]
- 46.Maksymowych WP, Dougados M, van der Heijde D, et al. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheum Dis 2015. doi:10.1136/annrheumdis-2014-eular.1138. [DOI] [PMC free article] [PubMed]
- 47.Song IH, Hermann K, Haibel H, et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): a 48-week randomised controlled trial. Ann Rheum Dis 2011; 70: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song IH, Althoff CE, Haibel H, et al. Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis 2012; 71: 1212–1215. [DOI] [PubMed] [Google Scholar]
- 49.Song IH, Weiß A, Hermann KG, et al. Similar response rates in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis after 1 year of treatment with etanercept: results from the ESTHER trial. Ann Rheum Dis 2013; 72: 823–825. [DOI] [PubMed] [Google Scholar]
- 50.Song I-H, Hermann KG, Haibel H, et al. Effective prevention of new osteitis on magnetic resonance imaging in patients with early axial spondyloarthritis during 3 years of continuous treatment with etanercept – data of the ESTHER trial. Presented at ACR 2013, abstract 1552. [DOI] [PubMed]
- 51.Sieper J, van der Heijde D, Dougados M, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013; 72: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van der Heijde D, Maksymowych WP, Sieper J, et al. Relationship between MRI and clinical remission in patients with non-radiographic axial spondyloarthritis after two years of adalimumab therapy. Presented at ACR 2013, abstract 1801.
- 53.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014; 73: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Heijde D, Maksymowych WP, Landewé R, et al. Effect of certolizumab pegol on inflammation of spine and sacroiliac joints in patients with axial spondyloarthritis: 12-week magnetic resonance imaging results of RAPID-axSpA study. Ann Rheum Dis 2013; 72(Suppl 3): 515–515. [Google Scholar]
- 55.Sieper J, Rudwaleit M, van der Heijde M, et al. Long-term safety and efficacy of certolizumab pegol in patients with axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis: 96-week outcomes of the RAPID-axSpA trial. Ann Rheum Dis 2014. doi:10.1136/annrheumdis-2014-eular.1636. Available at: http://www.abstracts2view.com/eular/view.php?nu=EULAR14L_SAT0351. [DOI] [PMC free article] [PubMed]
- 56.Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015; 67: 2702–2712. Epub ahead of print 2 July 2015. DOI: 10.1002/art.39257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Electronic Medicines Compendium. Enbrel: Summary of product characteristics. Updated 27 October 2014.
- 58.Electronic Medicines Compendium. Humira: Summary of product characteristics. Updated 10 September 2014.
- 59.Electronic Medicines Compendium. Cimzia: Summary of product characteristics. Updated 4 November 2014.
- 60.Electronic Medicines Compendium. Simponi: Summary of product characteristics. Updated 22 June 2015.
- 61.Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013; 72: 165–178. [DOI] [PubMed] [Google Scholar]
- 62.Plasencia C, Pascual-Salcedo D, Nuno L, et al. Influence of immunogenicity on the efficacy of longterm treatment of spondyloarthritis with infliximab. Ann Rheum Dis 2012; 71: 1955–1960. [DOI] [PubMed] [Google Scholar]
- 63.Kizza FN, List J, Nkwata AK, et al. Prevalence of latent tuberculosis infection and associated risk factors in an urban African setting. BMC Infect Dis 2015; 15: 165–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gottleib AB, Gordon K, Gianninni EH, et al. Clinical trial safety and mortality analyses in patients receiving etanercept across approved indications. J Drugs Dermatol 2011; 10: 289–300. [PubMed] [Google Scholar]
- 65.Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti–tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French research axed on tolerance of biotherapies registry. Arthritis Rheum 2009; 60: 1884–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tikly M, Njobvu P, McGill P. Spondyloarthritis in sub-Saharan Africa. Curr Rheumatol Rep 2014; 16: 421–421. [DOI] [PubMed] [Google Scholar]
- 67.Furst DE, Keystone EC, Fleischmann R, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2009. Ann Rheum Dis 2010; 69(Suppl 1): i2–i29. [DOI] [PubMed] [Google Scholar]
- 68.Hahn BH, Grossman J, Chen W, et al. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun 2007; 28: 69–75. [DOI] [PubMed] [Google Scholar]
- 69.Westhovens I, Lories RJ, Westhovens R, et al. Anti-TNF therapy and malignancy in spondyloarthritis in the Leuven spondyloarthritis biologics cohort (BIOSPAR). Clin Exp Rheumatol 2014; 32: 71–76. [PubMed] [Google Scholar]