Abstract
The small bowel is affected in the vast majority of patients with Crohn's Disease (CD). Small bowel capsule endoscopy (SBCE) has a very high sensitivity for the detection of CD-related pathology, including early mucosal lesions and/or those located in the proximal segments of the small bowel, which is a major advantage when compared with other small bowel imaging modalities. The recent guidelines of European Society of Gastrointestinal Endoscopy (ESGE) and European Crohn's and Colitis Organisation (ECCO) advocate the use of validated endoscopic scoring indices for the classification of inflammatory activity in patients with CD undergoing SBCE, such as the Lewis Score or the Capsule Endoscopy Crohn's Disease Activity Index (CECDAI). These scores aim to standardize the description of lesions and capsule endoscopy reports, contributing to increase inter-observer agreement and enabling a stratification of the severity of the disease. On behalf of the Grupo de Estudos Português do Intestino Delgado (GEPID) – Portuguese Small Bowel Study Group, we aimed to summarize the general principles and clinical applications of current endoscopic scoring systems for SBCE in the setting of CD, covering the topic of suspected CD as well as the evaluation of disease extent (with potential prognostic and therapeutic impact), evaluation of mucosal healing in response to treatment and evaluation of post-surgical recurrence in patients with previously established diagnosis of CD.
Keywords: Capsule Endoscopy, Crohn's Disease, Severity of Illness Index, Small Intestine
Resumo
O intestino delgado encontra-se envolvido pela doença na maioria dos pacientes com Doença de Crohn (DC). A enteroscopia por cápsula (EC) apresenta uma elevada sensibilidade na detecção de lesões relacionadas com a DC, incluindo as lesões superficiais mais precoces e/ou localizadas no segmentos proximais do intestino delgado, o que representa uma clara mais-valia comparativamente com os demais exames imagiológicos do intestino delgado. As recentes recomendações da ESGE (European Society of Gastrointestinal Endoscopy) e da ECCO (European Crohn's and Colitis Organisation) recomendam a utilização dos scores endoscópicos validados para a classificação da actividade inflamatória em doentes com DC submetidos a EC, nomeadamente o Score de Lewis ou o CECDAI (Capsule Endoscopy Crohn's Disease Activity Index). Estes scores permitem uniformizar a descrição das lesões e os relatórios em EC, contribuindo para uma melhoria da concordância entre observadores e possibilitando a estratificação da gravidade da doença. Em nome do GEPID (Grupo de Estudos Português do Intestino Delgado - Portuguese Small Bowel Study Group), os autores pretendem sumariar neste documento os princípios gerais e as aplicações clínicas actuais dos scores endoscópicos em EC no contexto da DC, incluindo quer a suspeita de DC, quer a avaliação da extensão da doença (com potencial impacto prognóstico e na decisão terapêutica), avaliação da cicatrização da mucosa em resposta ao tratamento e avaliação da recorrência pós-cirúrgica em doentes com um diagnóstico prévio de DC.
Palavras-chave: Enteroscopia por Cápsula, Doença de Crohn, Índice de Gravidade de Doença, Intestino Delgado
1. Introduction
The small bowel is affected in up to 80% of patients with Crohn's Disease (CD).1 Small bowel capsule endoscopy (SBCE) may contribute to establish the diagnosis in patients with suspected CD, often being the next step after an initial non-diagnostic ileocolonoscopy, if the patient has no history of occlusive symptoms, suspected stricturing or penetrating disease. The very high sensitivity of SBCE, inclusively for the detection of early superficial lesions and/or those located in the proximal segments of the small bowel, is a major advantage in the diagnostic evaluation of patients with suspected CD, when compared with other small bowel imaging modalities.2, 3
The recent guidelines of ESGE4 and ECCO1 recommend the use of validated endoscopic scoring indices for the classification of inflammatory activity in patients with CD undergoing SBCE, such as the Lewis Score5 or the Capsule Endoscopy Crohn's Disease Activity Index (CECDAI).6 These scores aim to standardize the description of lesions and capsule endoscopy reports, based on a structured terminology,7 thus providing a reproducible methodology for interpretation and estimation of endoscopic activity, while contributing for a higher inter-observer agreement. Moreover, these scores quantify objectively the inflammatory activity and enable a stratification of the severity of the disease. On behalf of the Grupo de Estudos Português do Intestino Delgado (GEPID) – Portuguese Small Bowel Study Group, this manuscript aims to summarize the general principles and current applications of the available endoscopic scoring systems for SBCE in the setting of CD.
2. Lewis Score
To calculate the Lewis Score, the small bowel is first divided into three equal parts (tertiles) based on capsule transit time from the first duodenal image to the first cecal image. For each tertile, a subscore is determined based on the extension and distribution of oedema (Fig. 1), as well as the number, size and distribution of ulcers (Fig. 2). The Lewis Score results of the sum of the worst affected tertile plus the score of stenosis (Fig. 3); stenoses are evaluated considering the entire length of the small bowel, independently of the division in tertiles – Table 1.
Figure 1.
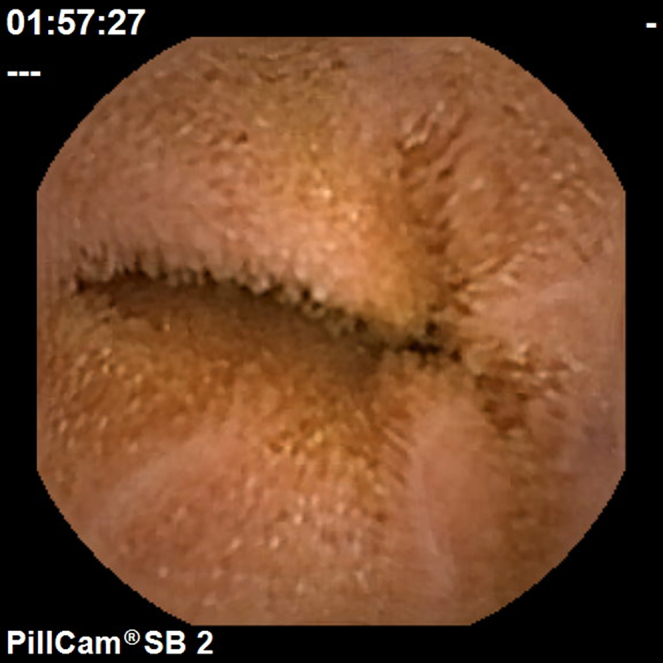
Villous edema.
Figure 2.
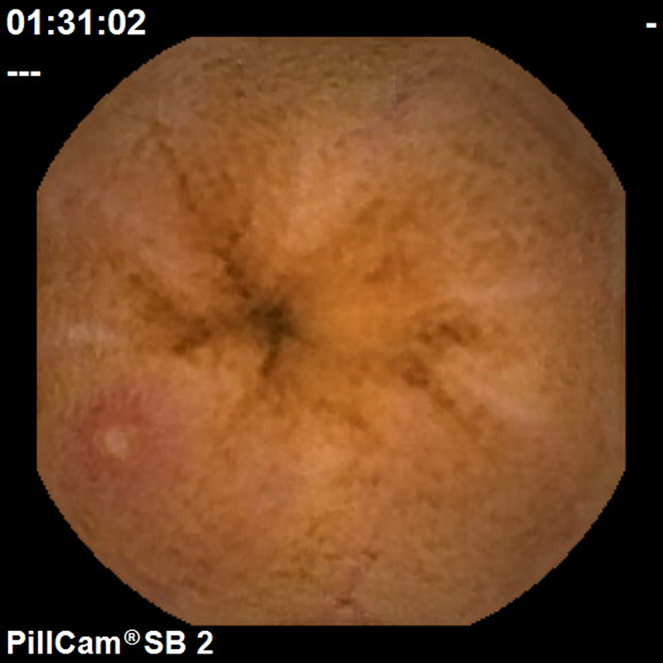
Aphthous ulcer.
Figure 3.
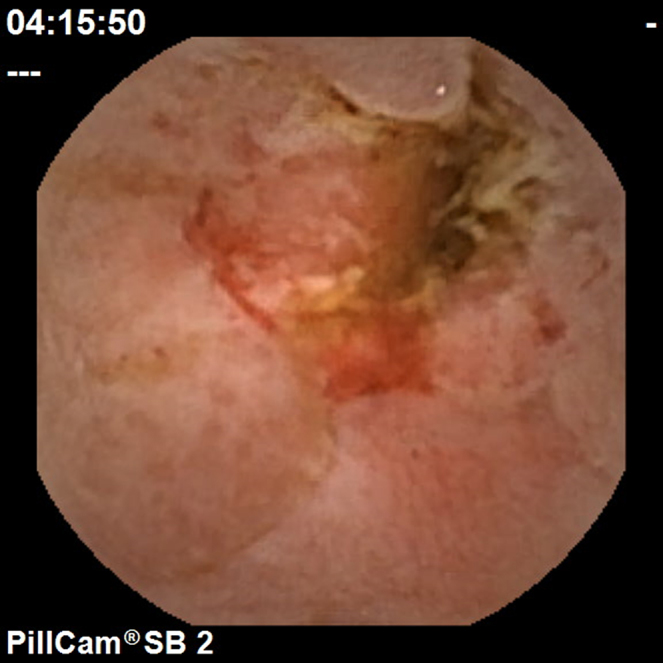
Ulcerated stricture.
Table 1.
Lewis Score.
| Number | Extent | Descriptors | |
|---|---|---|---|
| Villous appearance (worst-affected tertile) | Normal – 0 | ≤10% – 8 | Single – 1 |
| Oedematous – 1 | 11–50% – 12 | Patchy – 14 | |
| >50% – 20 | Diffuse – 17 | ||
| Ulcer (worst-affected tertile) | None – 0 | ≤10% – 5 | <1/4 – 9 |
| Single – 3 | 11–50% – 10 | 1/4–1/2 – 12 | |
| 2–7 – 5 | >50% – 15 | >1/2 – 18 | |
| ≥8 – 10 | (percentage of the frame occupied by the largest ulcer) ocupada | ||
| Stenosis (whole study) | None – 0 | Non-ulcerated – 2 | Traversed – 7 |
| Single – 14 | Ulcerated – 24 | Not traversed – 10 | |
| Multiple – 20 |
Lewis Score = tertile with highest score (resulting of oedema and ulcers) plus score of stenosis for the entire small bowel.
The Lewis Score has been recently validated for use in clinical practice.8 Although its calculation seems unpractical, a software has been developed to enable its automatic calculation, which has been incorporated into the RAPID READER® workstation of PillCam® capsules. Cut-off values have been devised to grade and classify small bowel inflammatory activity, as follows: Lewis Score <135 corresponds to a normal examination or non-significant inflammation; Lewis Score ≥135 and <790 corresponds to mild inflammation; and a Lewis Score ≥790 corresponds to moderate to severe activity.
3. Niv score or Capsule Endoscopy Crohn's Disease Activity Index (CECDAI)
The CECDAI evaluates three endoscopic parameters: inflammation (A, 0–5 points), extent of disease (B, 0–3 points) and strictures (C, 0–3 points), both for the proximal and distal segments of the small bowel based on the transit time of the capsule. The final score is calculated by adding the two segmental scores: proximal ([A1 × B1] + C1) + distal ([A2 × B2] + C2) – Table 2.
Table 2.
CECDAI (Niv Score).
| A. Inflammation score |
|---|
| 0 = None |
| 1 = Oedema/hyperemia/denudation (mild to moderate) |
| 2 = Oedema/hyperemia/denudation (severe) |
| 3 = Bleeding, exudate, aphthae, erosion, ulcer <0.5 cm |
| 4 = Ulcer 0.5–2 cm, pseudopolyp |
| 5 = Large ulcer >2 cm |
| B. Extent of disease score |
|---|
| 0 = No disease |
| 1 = Focal disease (single segment) |
| 2 = Patchy disease (2–3 segments) |
| 3 = Diffuse disease (>3 segments) |
| C. Stricture score |
|---|
| 0 = None |
| 1 = Single-passed |
| 2 = Multiple-passed |
| 3 = Obstruction (non-passage) |
CECDAI = proximal (A1 × B1 + C1) + distal (A2 × B2 + C2).
The CECDAI has been validated in a prospective multicentre trial.9 Currently, there is no software available for its automatic calculation; however, its calculation is quite easy, thus the CECDAI might eventually be more intuitive and preferable to the Lewis Score for physicians not using PillCam® capsules. No cut-off of inflammatory severity have been validated for the CECDAI, however the values of 3.8 and 5.8 have been pointed as approximately corresponding to the 135 and 790 cut-offs of the Lewis Score, respectively.10 The correlation of endoscopic activity indices with clinical activity scores, as well as serum or faecal inflammatory markers, is globally poor.11 Faecal calprotectin, which is usually considered as an accurate non-invasive marker of intestinal inflammation, has been shown to correlate more closely with the Lewis Score than with the CECDAI.10 This correlation, however, is only moderate12; indeed, while the Lewis Score performs relatively well in describing the paucity of small bowel inflammation at levels of faecal calprotectin <100 μg/g, at higher levels it seems to have little or no correlation, which means that patients may still benefit of capsule endoscopy to assess for small bowel lesions activity and extension.
4. Practical clinical applications
4.1. Suspected Crohn's disease
Small bowel capsule endoscopy (SBCE) has a very high negative predictive value in patients with suspected Crohn's Disease (CD) and non-diagnostic ileocolonoscopy, such that when the capsule is negative, a diagnosis of CD can be confidently excluded in up to 96% of cases.13 Conversely, the detection of lesions which are suggestive of CD is usually a key element to establish the diagnosis, if there is a consistent clinical context and if non-steroidal anti-inflammatory drugs (NSAIDs) consumption has been adequately excluded for at least one month prior to the capsule.14 However, it is important to underline that endoscopic findings are non-specific, no single lesion is pathognomonic of CD, and there is no cut-off value of any endoscopic scoring system above which the diagnosis can be firmly established; indeed, the scores are not discriminative, since they grade inflammatory activity independently from its aetiology. Nonetheless, some studies have shown that small bowel CD is unlikely when the Lewis Score at SBCE is under 135.8, 14, 15 Alternative diagnoses such as Behçet's Disease, tuberculosis, intestinal lymphoma or vasculitis should also be considered and excluded whenever in doubt; in some cases, deep enteroscopy may be valuable to further characterize and take biopsies of the lesions previously identified by the capsule, although this seems not to be routinely necessary.1, 4 The criteria indicated by the International Conference on Capsule Endoscopy (ICCE)16 aim to optimise the adequate selection of candidates for SBCE among those patients with clinical suspicion of CD. In fact, in order to avoid an overdiagnosis of CD it is important that the interpretation of the lesions detected at SBCE takes into account the pre-test probability of CD, which according to the above mentioned ICCE criteria should be based on a conjunction of clinical, analytical and/or imaging suggestive features.
4.2. Established Crohn's disease
In patients with previously known CD, the initial evaluation of the small bowel should be performed with cross sectional imaging such as CT or MR enterography (CTE or MRE), to allow for a transmural assessment of disease activity, enabling the identification and characterization of strictures, as well as any evidence of extra-luminal disease and complications such as fistulae or abscesses.1 SBCE may be useful in some specific clinical scenarios in established CD; however, it should be preceded by small bowel cross sectional imaging and/or a patency capsule to minimize the risk of capsule retention.
4.2.1. Investigation of persistent symptoms
The very high sensitivity of SBCE, even for subtle mucosal lesions, may help to determine if symptoms such as abdominal pain or persistent diarrhoea are in relation with CD activity, or may be otherwise attributable to other concurrent diagnoses, such as irritable bowel syndrome, poor absorption of bile acids, exocrine pancreatic insufficiency or bacterial overgrowth, among others.17
4.2.2. Evaluation of disease extent
The existence of extensive small bowel involvement and/or proximally located lesions are both poor prognostic factors in CD; such findings may influence therapeutic decisions, namely by triggering an earlier initiation of immunosuppressive medication in CD.18, 19, 20, 21, 22 Some recent studies have pointed to the existence of proximal small bowel lesions in up to 50% of patients with CD, and in many cases those are missed by prior small bowel imaging.19, 21 Thus, performing a SBCE should be considered at diagnosis if the clinician considers that the finding of new lesions in the small bowel, not evident in prior cross sectional imaging, could influence treatment decision. In a recent study,21 patients with a previous diagnosis of CD were submitted to SBCE; the endoscopic findings directly triggered a therapeutic escalation with initiation of immunosuppressive treatment, especially when lesions were located in the upper segments of the small bowel (6%, 36% and 45% of patients with non-significant, mild and moderate-to-severe lesions, respectively). In another study, treatment was intensified after SBCE in 14.5%, 48.1% and 87.1% of patients with Lewis Score <135, Lewis Score 135–790 and Lewis Score≥790, respectively.11
4.2.3. Evaluation of mucosal healing
SBCE enables the longitudinal assessment of small bowel mucosal healing, which is an important endpoint of treatment efficacy in CD. The use of validated endoscopic scores, such as the Lewis Score or the CECDAI, may be particularly interesting in this setting, by enabling an objective quantification of the evolution of inflammatory activity, although standard criteria of mucosal improvement and mucosal healing are yet to be established. In a recent prospective study, symptomatic and biochemical response to treatment appeared to be mirrored by endoscopic remission in only 42% of patients with active small bowel CD.23 Although there are currently no formal recommendations for the assessment of mucosal healing with SBCE in clinical practice, some groups have already started exploring this issue, and it is becoming an interesting topic for future investigation.23, 24, 25
4.2.4. Evaluation of post-surgical recurrence
Ileocolonoscopy, with routine assessment of the Rutgeerts score, remains the gold standard for the evaluation of post-surgical recurrence in CD. The percentage of agreement between ileocolonoscopy and SBCE for the detection of lesions in the neo-terminal ileum has been widely variable in the literature,26, 27 and the prognostic value of the different grades of inflammatory activity detected by the capsule is currently unknown; moreover, although the capsule may detect more lesions in the upper small bowel in a significant proportion of these patients, its clinical significance and prognostic value are currently a matter of debate, requiring further investigation.
5. Conclusions
SBCE is a useful diagnostic examination, either for the diagnosis and follow-up of patients with CD. The routine use of quantitative endoscopic indices of severity of inflammatory activity has been encouraged by scientific societies, as it may contribute to improve the interpretation of lesions, reducing inter-observer variability and enabling solid comparative data analysis between different patients and between different studies. Thus, it seems very important that the use of a validated endoscopic scoring index should be systematically considered in future studies, potentially influencing in a near future our practical approach either for diagnosis, staging, prognostic evaluation, therapeutic decision and follow-up of patients with CD.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Annese V., Daperno M., Rutter M.D., Amiot A., Bossuyt P., East J. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohn's Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Dionisio P.M., Gurudu S.R., Leighton J.A., Leontiadis G.I., Fleischer D.E., Hara A.K. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn's disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240–1248. doi: 10.1038/ajg.2009.713. [DOI] [PubMed] [Google Scholar]
- 3.Jensen M.D., Nathan T., Rafaelsen S.R., Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn's disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124–129. doi: 10.1016/j.cgh.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Pennazio M., Spada C., Eliakim R., Keuchel M., May A., Mulder C.J. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352–386. doi: 10.1055/s-0034-1391855. [DOI] [PubMed] [Google Scholar]
- 5.Gralnek I.M., Defranchis R., Seidman E., Leighton J.A., Legnani P., Lewis B.S. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146–154. doi: 10.1111/j.1365-2036.2007.03556.x. [DOI] [PubMed] [Google Scholar]
- 6.Gal E., Geller A., Fraser G., Levi Z., Niv Y. Assessment and validation of the new capsule endoscopy Crohn's disease activity index (CECDAI) Dig Dis Sci. 2008;53:1933–1937. doi: 10.1007/s10620-007-0084-y. [DOI] [PubMed] [Google Scholar]
- 7.Korman L.Y., Delvaux M., Gay G., Hagenmuller F., Keuchel M., Friedman S. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy. 2005;37:951–959. doi: 10.1055/s-2005-870329. [DOI] [PubMed] [Google Scholar]
- 8.Cotter J., Dias de Castro F., Magalhaes J., Moreira M.J., Rosa B. Validation of the Lewis score for the evaluation of small-bowel Crohn's disease activity. Endoscopy. 2015;47:330–335. doi: 10.1055/s-0034-1390894. [DOI] [PubMed] [Google Scholar]
- 9.Niv Y., Ilani S., Levi Z., Hershkowitz M., Niv E., Fireman Z. Validation of the Capsule Endoscopy Crohn's Disease Activity Index (CECDAI or Niv score): a multicenter prospective study. Endoscopy. 2012;44:21–26. doi: 10.1055/s-0031-1291385. [DOI] [PubMed] [Google Scholar]
- 10.Koulaouzidis A., Douglas S., Plevris J.N. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn's Disease Activity Index. Dig Dis Sci. 2012;57:987–993. doi: 10.1007/s10620-011-1956-8. [DOI] [PubMed] [Google Scholar]
- 11.Kopylov U., Nemeth A., Koulaouzidis A., Makins R., Wild G., Afif W. Small bowel capsule endoscopy in the management of established Crohn's disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis. 2015;21:93–100. doi: 10.1097/MIB.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 12.Koulaouzidis A., Nemeth A., Johansson G.W., Toth E. Dissecting Lewis score under the light of fecal calprotectin; an analysis of correlation of score components with calprotectin levels in capsule endoscopy. Ann Gastroenterol. 2015;28:259–264. [PMC free article] [PubMed] [Google Scholar]
- 13.Tukey M., Pleskow D., Legnani P., Cheifetz A.S., Moss A.C. The utility of capsule endoscopy in patients with suspected Crohn's disease. Am J Gastroenterol. 2009;104:2734–2739. doi: 10.1038/ajg.2009.404. [DOI] [PubMed] [Google Scholar]
- 14.Rosa B., Moreira M.J., Rebelo A., Cotter J. Lewis Score: a useful clinical tool for patients with suspected Crohn's Disease submitted to capsule endoscopy. J Crohn's Colitis. 2012;6:692–697. doi: 10.1016/j.crohns.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro S., Carvalho P.B., Dias de Castro F., Magalhaes J., Machado J., Moreira M.J. Capsule endoscopy: diagnostic accuracy of Lewis Score in patients with suspected Crohn's Disease. Inflamm Bowel Dis. 2015;21:2241–2246. doi: 10.1097/MIB.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 16.Mergener K., Ponchon T., Gralnek I., Pennazio M., Gay G., Selby W. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy. 2007;39:895–909. doi: 10.1055/s-2007-966930. [DOI] [PubMed] [Google Scholar]
- 17.Mehdizadeh S., Chen G.C., Barkodar L., Enayati P.J., Pirouz S., Yadegari M. Capsule endoscopy in patients with Crohn's disease: diagnostic yield and safety. Gastrointest Endosc. 2010;71:121–127. doi: 10.1016/j.gie.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Van Assche G., Dignass A., Panes J., Beaugerie L., Karagiannis J., Allez M. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohn's Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Flamant M., Trang C., Maillard O., Sacher-Huvelin S., Le Rhun M., Galmiche J.P. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn's disease. Inflamm Bowel Dis. 2013;19:1390–1396. doi: 10.1097/MIB.0b013e31828133c1. [DOI] [PubMed] [Google Scholar]
- 20.Park S.K., Yang S.K., Park S.H., Kim J.W., Yang D.H., Jung K.W. Long-term prognosis of the jejunal involvement of Crohn's disease. J Clin Gastroenterol. 2013;47:400–408. doi: 10.1097/MCG.0b013e3182705f9e. [DOI] [PubMed] [Google Scholar]
- 21.Cotter J., Dias de Castro F., Moreira M.J., Rosa B. Tailoring Crohn's disease treatment: the impact of small bowel capsule endoscopy. J Crohn's Colitis. 2014;8:1610–1615. doi: 10.1016/j.crohns.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Dias de Castro F., Boal Carvalho P., Monteiro S., Rosa B., Moreira M.J., Cotter J. Lewis Score – prognostic value in patients with isolated small bowel Crohn's disease. J Crohn's Colitis. 2015;9:S125. doi: 10.1093/ecco-jcc/jjv166. [DOI] [PubMed] [Google Scholar]
- 23.Hall B., Holleran G., Chin J.L., Smith S., Ryan B., Mahmud N. A prospective 52 week mucosal healing assessment of small bowel Crohn's disease as detected by capsule endoscopy. J Crohn's Colitis. 2014;8:1601–1609. doi: 10.1016/j.crohns.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Dulai P.S., Levesque B.G., Feagan B.G., D’Haens G., Sandborn W.J. Assessment of mucosal healing in inflammatory bowel disease: review. Gastrointest Endosc. 2015;82:246–255. doi: 10.1016/j.gie.2015.03.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boal Carvalho P., Rosa B., Dias de Castro F., Moreira M.J., Cotter J. PillCam COLON 2(©) in Crohn's disease: a new concept of pan-enteric mucosal healing assessment. World J Gastroenterol. 2015;21:7233–7241. doi: 10.3748/wjg.v21.i23.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pons Beltran V., Nos P., Bastida G., Beltran B., Arguello L., Aguas M. Evaluation of postsurgical recurrence in Crohn's disease: a new indication for capsule endoscopy? Gastrointest Endosc. 2007;66:533–540. doi: 10.1016/j.gie.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 27.Bourreille A., Jarry M., D’Halluin P.N., Ben-Soussan E., Maunoury V., Bulois P. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn's disease: a prospective study. Gut. 2006;55:978–983. doi: 10.1136/gut.2005.081851. [DOI] [PMC free article] [PubMed] [Google Scholar]


