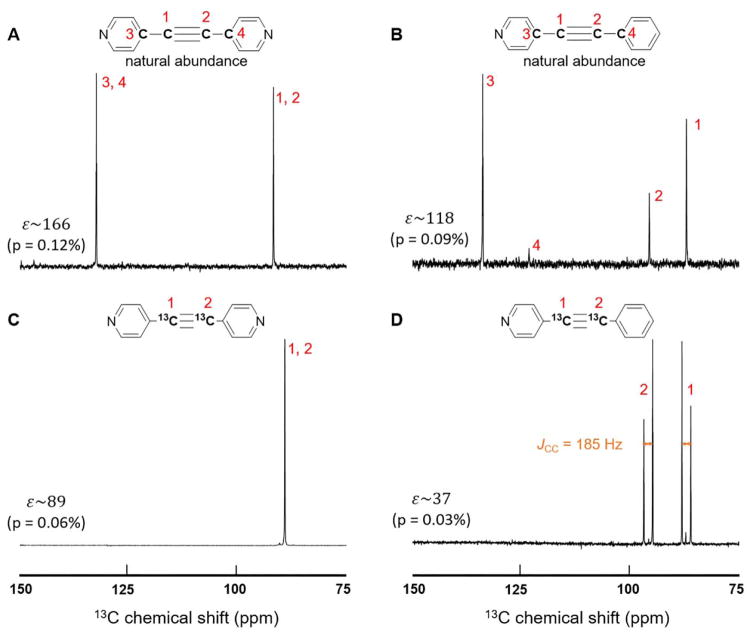
Figure 1.
13C spectra of naturally abundant (A&B) and 13C labelled (C&D) substrates used in experiments. A&C show results for the symmetrically substituted 1,2-2 pyridyl acetylene. B&D are from the asymmetrically substituted 1-phenyl-2-(4-pyridyl) acetylene. For the naturally abundant substrates the bridge carbons on the pyridyl rings (3, 4 in A, 3 in B) show significant enhancement, while the one on the benzene ring (4 in B) is only slightly hyperpolarized. The 13C-13C coupling, JCC, read from the line-splitting in panel D is 185 Hz. (The SI also provides a thermal spectrum in Fig. S2.)