Fig. 5. Analysis of Tim50IMS-bilayer interactions from CG MD simulations.
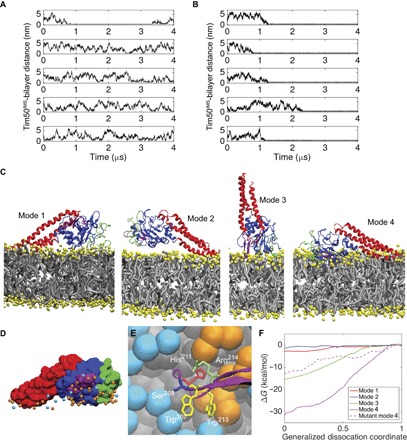
(A and B) Time course profiles of the Tim50IMS-bilayer distance. The minimum distance between Tim50IMS and the bilayers composed of pure POPC (A) or POPC with 20 mol % TOCL (B) is shown for five MD simulations each. (C) Four unique modes of Tim50IMS binding to TOCL-containing bilayers. N-terminal region, green; Tim50CORE, blue; β-hairpin, purple; C-terminal region, red; lipid headgroups, yellow; lipid hydrocarbon chains, gray. (D) Lipid headgroup phosphate contacts with Tim50IMS in binding mode 4. Tim50IMS is shown in space-filling representation with protein coloring the same as that in (C). POPC phosphates, cyan; TOCL phosphates, orange. (E) Tim50IMS β-hairpin–bilayer interactions in binding mode 4. Detailed image of key β-hairpin side chains shown to stabilize the Tim50IMS-bilayer interaction (W207, H211, W213, and R214) and the site of extrinsic fluorescent probe attachment (S208; see Fig. 7). POPC headgroup beads, cyan; TOCL headgroup beads, orange; lipid hydrocarbon tails, gray. (F) Free energy binding curves. Umbrella sampling results are shown for the four unique binding modes as well as for the four-point mutant of mode 4. The abscissa is a normalized coordinate such that x = 0 when Tim50IMS is bound and x = 1 when Tim50IMS is fully dissociated. This coordinate was used for clarity because the different modes required different absolute displacements from the bilayer to become fully dissociated.
