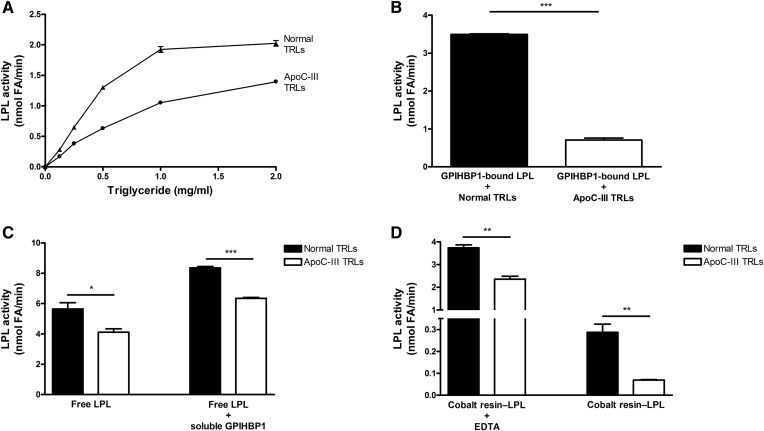
Fig. 4.
ApoC-III–enriched TRLs are suboptimal substrates for LPL, particularly when LPL is immobilized on GPIHBP1. A: LPL-mediated triglyceride hydrolysis with normal TRLs and apoC-III–TRLs. Increasing amounts of normal TRLs (closed triangles) and apoC-III–TRLs (closed circles) were added to bovine LPL (10 nM), and LPL activity was measured. B: A comparison of the processing of normal TRLs and apoC-III–TRLs by GPIHBP1-bound LPL. Normal TRLs and apoC-III–TRLs were incubated with bovine LPL that was bound to GPIHBP1 on agarose beads. C: Processing of normal TRLs and apoC-III–TRLs by free LPL and free LPL–GPIHBP1 complexes (i.e., LPL bound to soluble GPIHBP1). LPL was incubated on ice for 10 min with or without soluble GPIHBP1. Aliquots of those samples were then mixed with normal TRLs or apoC-III–TRLs and LPL activity was measured. D: A comparison of the processing of normal TRLs or apoC-III–TRLs by V5–His-tagged LPL immobilized on cobalt resin, both in the presence and absence of EDTA. The LPL was incubated in microtiter plates in (A) and in microfuge tubes in (B–D). LPL activity was determined by measuring the production of FFAs (see Materials and Methods). Mean ± SEM (n = 3 experiments). *P < 0.05; **P < 0.01; ***P < 0.001.