Abstract
Dry eye is a common, multifactorial disease currently diagnosed by a combination of symptoms and signs. However, the subjective symptoms of dry eye poorly correlate to the current gold standard for diagnostic tests, reflecting the need to develop better objective tests for the diagnosis of dry eye. This review considers the role of ocular surface matrix metalloproteinase 9 (MMP-9) in dry eye and the implications of a novel point-of-care test that measures MMP-9 levels, InflammaDry (RPS, Sarasota, FL) on choosing appropriate therapeutic treatments.
Keywords: dry eye, InflammaDry, matrix metalloproteinase 9
I. Introduction
Dry eye (DE) is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear instability.1 DE is a common ocular condition that significantly reduces quality of life and affects 5–30% of the population.2 Given the ubiquity of DE and its impact on patients’ quality of life (i.e., patients consider the effects of DE to be equivalent to those of moderate to severe angina), one would think that a plethora of effective screening tests and treatment options would be available.3 Yet, only about two-thirds of patients reporting DE symptoms test positive for DE with existing confirmatory tests.4, 5 Furthermore, none of the available screening tests have been shown to predict the course of DE or its response to treatment. For example, lubricating eye drops remain the first-line therapy for DE, but it is not known which patients will not respond and will require further therapy.
Inflammation is known to be an important component of DE pathophysiology, but it is not known if all patients with DE have ongoing inflammation and would thus be good candidates for anti-inflammatory therapy. In a multicenter, randomized, double-masked, vehicle-controlled study, only 39% (n=115) of patients treated with topical cyclosporine emulsion 0.1% for 6 months had at least a moderate response to treatment versus 32% in the vehicle group (n=93), as determined by the physician’s subjective assessment of global response to treatment.6 In another prospective study of patients with delayed tear clearance, nonpreserved topical methylprednisolone (3 times daily for 3 weeks) improved signs of ocular surface inflammation in some but not all patients. Specifically, 80% (n=56) of patients demonstrated a reduction in bulbar conjunctival injection, decreased vital dye staining, and/or reduction of a tarsal papillary response after treatment.7
The response of DE to anti-inflammatory treatment in prior studies suggests that inflammation is a key component of DE, but the fact that not all patients respond suggests that perhaps not all DE patients have significant inflammation requiring anti-inflammatory treatment. Interestingly, while it is difficult to directly compare study results, because of differences in study designs, the frequency of response was higher after corticosteroid treatment than after cyclosporine treatment. These findings reflect the complexity and multifactorial etiology of DE and underscore the necessity for better diagnostic tests that can guide management options. Detection of elevated MMP-9 on the ocular surface may be one such test that can aid in appropriate therapeutic selection.
II. Matrix Metalloproteinase 9 (MMP-9)
MMP-9 is a 23 zinc and calcium ion-dependent enzyme important for tissue remodeling in normal physiological processes like wound healing and bone development.8 MMP-9 is also understood to play a pathogenic role in inflammatory disease, arthritis, cardiovascular diseases, pulmonary diseases, and cancer.
MMP-9 activity is regulated by epigenetic processes, cell-cell interactions, and cytokine-mediated pathways. On the corneal surface, the hyperosmolarity of the tear fluid seen in DE has been shown to trigger the stress-activated protein kinase (SAPK) signaling cascade. SAPK signaling leads to the release of MMP-9 from corneal epithelial cells themselves, thus initiating a cycle of progressive inflammation.9–13 Tight junction proteins occludin and zonula occludens-1 (ZO-1) are cleaved by MMP-9, thereby disrupting epithelial layers.14
Additionally, mediators such as interleukin (IL)-1β derived from mitogen-activated protein kinases (MAPK) and nuclear factor (NF)-kβ pathways bind to the mmp-9 gene promotor region of local neutrophils where MMP-9 is constitutively stored in vesicles.15 MMP-9 is then secreted in its proenzyme form bound to endogenous tissue inhibitors of metalloproteinases (TIMPs) and activated extracellularly by other proteinases.16 MMP-9, in turn, activates other inflammatory tear fluid factors like pro-IL-1 beta, pro-tumor necrosis factor (TNF)-alpha, and substance P. MMP-9 may also play a role in processing molecules that initiate a positive feedback loop to increase EGFR signals to increase cell migration and upregulate MMP-9 expression.15
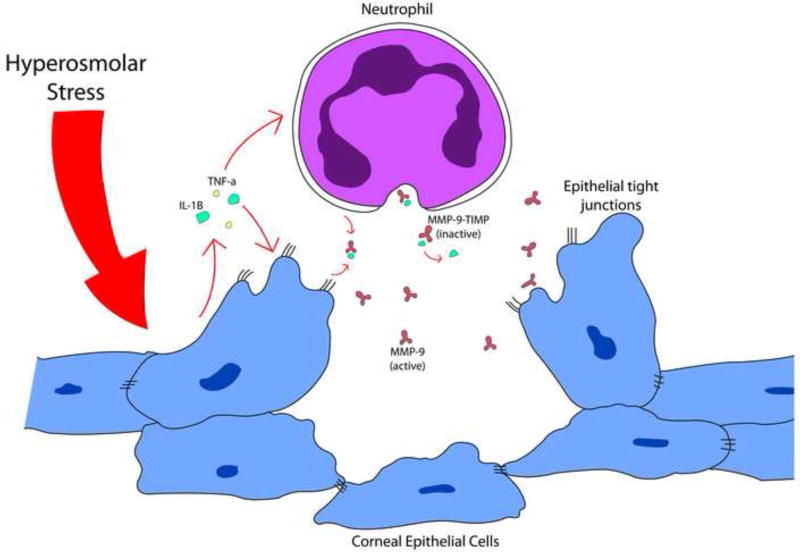
In summary, T-cell recruitment, the proteolytic activity of the MMP-9 molecule itself, and activation of secretion of additional cytokines initiate a self-perpetuating cycle of inflammation, secretory dysfunction, corneal surface irregularity, and worsening eye dryness (Figure 1).14, 17
Figure 1.
Hyperosmolar stress to the ocular surface causes the release of MMP-9-TIMP complexes from neutrophils, which when released from bound inactivating TIMP disrupt corneal surface epithelial junctions.
III. MMP-9 in Dry Eye
Much evidence has been found in support of MMP-9’s role in DE from experimental dry eye (EDE) mouse models. Mice subjected to scopolamine hydrobromide injections four times per day and exposure to a low humidity air draft for 18 hours per day display an EDE phenotype consisting of corneal staining, digestion of tight junction proteins, and a rise in MMP-9 activity.
In a study by Corrales et al, tear fluid washings extracted by capillary tubes from the tear meniscus of EDE mice displayed significantly greater MMP-9 activity on an MMP activity assay (Biotrack; GE Healthcare) than controls 4 days after induction of EDE (P <.001). Corneal epithelial tissue samples from EDE and control mice were also tested for MMP-9 transcripts by real-time polymerase chain reaction (PCR) and MMP-9 activity by the MMP activity assay. MMP-9 immunofluorescence, transcripts, and activity from the corneal epithelial tissue were all shown to increase 5–10 days after EDE induction compared to control mice. In this model, corneal permeability to Oregon green dextran (OGD) and sodium fluorescein was also elevated compared to controls (P <.001), suggesting that MMP-9 may play a role corneal barrier disruption.18
Using the EDE mouse model, Luo et al confirmed an increase in MMP-9 activity using gel zymography assays on tear fluid washings of EDE mice treated for 10 days, which was not present before treatment. Corneal epithelial tissue samples contained higher levels of MMP-9 mRNA transcripts on semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) after 5 and 10 days of EDE treatment compared to controls.19 In another study by Luo et al, mice treated with hyperosmolar saline drops 6 times a day for 2 days had increased MMP-9 transcripts on RT-PCR of corneal tissue samples as well as an elevation of other inflammatory cytokines.20
Conversely, MMP-9 knockout mice were found to be resistant to developing the EDE phenotype.9 However, when MMP-9 knockout mice exposed to EDE conditions were treated with topical MMP-9 to the ocular surface, epithelial sloughing was observed. No sloughing was seen, however, when MMP-9 was administered to these knockout mice without exposure to EDE conditions.14 Immunofluorescence of apical corneal epithelia for tight junction protein occludin, a substrate of MMP-9, was also diminished in wild type mice exposed to EDE conditions but intact in the MMP-9 knockout mice.
Elevated MMP-9 levels have also been detected in DE patients. For reference, normal levels of MMP-9 (ng/ml) in human tears range from 3 to 41 ng/ml with 90% of people less than 30 ng/ml.21 Chotikanovich et al measured tear MMP-9 activity in 46 patients newly diagnosed with DE (as defined by an ocular surface disease index (OSDI) score >20 with one or more of the following: tear film breakup time (TFBUT)≤7 seconds, punctate corneal fluorescein staining, or Schirmer I score <10 mm) and 19 asymptomatic controls.8 Subjects were excluded if they were using any topical medications or had another ocular surface disease. An MMP activity assay kit (Biotrak; Amersham Biosciences, Piscataway, NJ) quantified MMP activity from a 0.1 µL tear sample collected with a capillary tube from the inferior tear meniscus based on the sample’s ability to activate a modified prodetection enzyme. DE patients stratified to the lowest severity DE group based on DE symptom severity scores had a significantly increased MMP-9 activity of 35.57 ± 17.04 ng/mL (P <.008) as compared to controls (8.39 ± 4.70 ng/mL). DE patients in the highest severity category had a mean MMP-9 activity of 381.24 ± 42.83 ng/mL (P <.001), which was significantly increased compared to controls and all other DE severity groups.
Within this same study, a subset of 19 of the DE patients and 16 controls were recruited for evaluation of mmp-9 gene expression in the conjunctival epithelium. Samples were collected by impression cytology of the inferotemporal bulbar conjunctiva, mRNA isolated by binding to a silica-gel-based membrane (RNeasy Micro kit; Qiagen, Gaithersburg, MD), and concentration determined by quantitative RT PCR. Patients with DE were found to have 8.25 ± 3.10 (P <.002) times as many MMP-9 mRNA transcripts as compared to controls.8
VanDerMeid et al compared MMP-9 concentrations in 30 healthy subjects with four standard DE diagnostic tests: Schirmer test, TFBUT, tear osmolarity, and OSDI. Subjects were included in the study if they were 18 years of age or older, had no allergic conjunctivitis, and were not using ophthalmic drops 8 hours prior to study visit, topical ocular medications, or contact lenses. MMP-9 and cytokines were extracted from tear samples using a novel method of recovery from Schirmer strips and quantified by a bead-based multiplex assay (Luminex, Austin, TX).22 MMP-9 levels were found to increase as Schirmer strip measurement decreased and tear osmolarity increased as determined by a Spearman correlation (P <.05), but no correlation was found for TFBUT or OSDI. Of note, only 5 of the 30 subjects had DE as defined by a tear osmolarity of >308 mOsm/L.23
Subjects with DE secondary to other diseases also have a strong association with elevated MMP-9 activity. Solomon et al found that tear fluid MMP-9 activity levels were significantly elevated in 13 patients with meibomian gland disease (MGD), defined as: 1) Schirmer >5 mm in each eye, 2) lid hyperemia or conjunctival hyperemia or 30% atrophy of meibomian gland acini in the lower lid, and 3) skin rosacea24) and in 9 Sjogren syndrome (SS) patients compared to 17 controls (defined as Schirmer test value >15 mm, normal meibomian glands, no ocular irritation symptoms, and no corneal fluorescein staining), P <.001.25 Other studies similarly found elevated MMP-9 activity in patients with conjunctivochalasis, vernal keratoconjunctivitis, keratoconus, and extended contact lens wear.26–30
To summarize, these studies suggest that MMP-9 production increases in response to hyperosmolar conditions, contributes to corneal barrier disruption, and rises with increasing levels of DE severity. MMP-9, however, has been shown not to be specific for a certain subtype of DE but is present in a wide range of ocular surface conditions. Finally, MMP-9 levels demonstrate variable agreement with DE tests in current use.
IV. MMP Levels with Dry Eye Treatment
Anti-inflammatory corticosteroids, cyclosporine, and tetracycline derivatives are agents known to reduce tear fluid MMP-9 levels.31–35 In a study by De Paiva et al, EDE mice were either left untreated or treated with topical doxycycline, tetracycline, or saline solution four times a day. MMP-9 expression was significantly diminished in the tetracycline- or doxycycline-treated mice as compared to the untreated mice (P <.001). Saline-treated mice also experienced a reduction in MMP-9 expression, but to a lesser degree (P <.05).31
A novel, synthetic molecule, PES_103, developed by Mori et al was shown to inhibit MMP-9 in vitro. Scopolamine patches were applied to mouse tails to induce a cholinergic blockade, then treated topically four times a day for 5 days with PES_103. Tear volume decreased and MMP-9 activity increased with induction of the cholinergic blockade. With administration of PES_103, both tear volume and MMP-9 activity were restored to physiological levels.32
MMP-9 activity has been shown to diminish in human corneal epithelial cultures treated with either methylprednisolone or doxycycline. Cultured corneal epithelial cells incubated with doxycycline for 24 hours showed a 70% decrease in supernatant MMP-9 activity on gel zymography as compared to untreated controls (P <.005). Treatment with methylprednisolone also showed a significant reduction in MMP-9 activity as compared to controls (P <.006).33
In humans, Pflugfelder et al conducted a randomized controlled trial of 64 patients with a DE diagnosis of at least 6 months’ duration, and all of the following criteria in at least one eye: 1) delayed tear clearance defined by a standardized visual scale score of ≥336; 2) at least one symptom >30 mm on visual analog scale; and 3) composite corneal staining score of ≥ 3. Subjects were excluded from the study if they had a current ocular infection or a history of herpes simplex infection, were using contact lenses, had had punctal occlusion within the past 3 months, had used topical corticosteroid or ophthalmic medication the last 2 months, or had had ophthalmic surgery in the past 6 months. Patients were given a 0.5% suspension of the corticosteroid loteprednol or placebo drop administered four times a day for 4 weeks. After 2 weeks, patients with moderate-to-severe DE symptoms treated with corticosteroid drops were shown to have significant improvements in corneal staining tests, conjunctival hyperemia, and lid margin injection.34 These improvements may have been mediated by a reduction in MMP-9 levels, which is known to occur with corticosteroid use. However, MMP-9 levels were not directly measured in this study, and thus the relationships between MMP-9 levels, DE metrics, and treatment response are not known.
Further data to support anti-inflammatory treatment for DE comes from a case-controlled study of 75 female subjects. Aragona et al compared MMP-9 expression in subjects treated with saline versus subjects treated with saline plus topical 0.5% loteprednol etabonate eye drops four times daily for 15 days. Thirty subjects had SS (defined by American-European Consensus Group International Classification Criteria), 30 subjects had MGD (defined by the presence of one or more of the following: 1) vascular engorgement, 2) anterior or posterior lid displacement of mucocutaneous junction, 3) irregularity of the lid margin, or 4) turbid meibum), and 15 were healthy controls. Exclusion criteria were: Schirmer I test results less than 10 mm in 5 minutes in the MGD group; systemic diseases other than SS or SS that had changed clinically in the last month; ocular diseases other than DE; and systemic or topical medications that could interfere with tear production. MMP-9 expression was measured by RT-PCR, and subjects with SS and MGD were found to have a 66% and 42% reduction in MMP-9 expression, respectively, from baseline levels.9
Topical cyclosporine (an inhibitor of T-cell proliferation and apoptosis of ocular surface cells37) has also been shown to reduce MMP-9 levels.38 In a study by Gürdal et al, 13 Grave’s disease patients with thyroid orbitopathy and DE (defined as Schirmer tear test <10mm/5 min in both eyes or TFBUT <10sec in both eyes) were treated for 2 months with topical cyclosporine A 0.05% (Restasis; Allergan, Irvine, CA). DE symptoms in all 13 patients were diminished after 2 months (mean OSDI decreased from 58.08 ± 6.28 to 36.41 ± 11.75, P=.001). Mean TFBUT also improved from 3.92 ± 2.18 seconds to 9.16 ± 3.34 seconds (P=.001), and the apoptosis rate of conjunctival epithelial cells decreased from 72.10 ± 35.82% to 53.29 ± 34.46% (P=.008). MMP-9 expression of conjunctival epithelial cells decreased from 48.12 ± 28.58% to 26.66 ± 25.13% (P=.005) is determined by cytoplasmic staining. This study was the first to show the inhibition of MMP-9 expression after treatment with cyclosporine.38 To summarize, treatments designed to reduce inflammation and, ostensibly, inflammatory markers such as MMP-9, reduce signs and symptoms of DE.
V. InflammaDry Test
InflammaDry is a novel test that measures the presence of MMP-9 (both the proform and active MMP-9 on the ocular surface). The test should be performed prior to administering ocular anesthetic, topical dyes, or Schirmer test. Tear samples are collected from the palpebral conjunctiva with the sample collector fleece until it glistens, indicating that the sampling fleece is saturated. The sampling fleece is then placed within the test cassette with the addition of buffer solution. Within 10 minutes, if there is an MMP-9 antibody-antigen interaction on the immunoassay test strip, the result window will read positive with two lines (one blue and the other one red). The test provides a qualitative (yes/no) response. According to the manufacturer, the intensity of the red line is directly related to the amount of MMP-9 present. The lower detection limit of the test is 40 ng/ml, which means that 100% of people can see a positive result at 40 ng/ml; however, a significant number of patients will still be perceived positive with faint positive lines between 30ng/ml and 40ng/ml. If no interaction occurs, only the blue line is seen. Proper specimen acquisition is vital to the accuracy of the test. If the collected sample is less than 5 µL, the test may falsely give a negative result.
As MMP-9 is a nonspecific marker, many conditions other than DE can produce a positive result. This information is found in the package insert and includes conditions such as recent ocular surgery, infection, or allergic conjunctivitis. The package insert further states that false negative results can occur in the setting of systemic immunomodulators, topical or oral steroids, cyclosporine, tetracycline, and topical azithromycin, all of which may inhibit metalloprotease activity. The test should be avoided in patients with cicatricial conditions that could lead to conjunctival injury or allergies to cornstarch, Dacron, topical anesthetic, or fluorescein dye.
VI. Published Findings on Use of InflammaDry in Evaluation of Dry Eye
A few studies have evaluated the relationship between InflammaDry and other metrics of DE. In a study by Samburksy et al, 206 subjects were recruited and given a clinical diagnosis of DE if they had an OSDI ≥13, Schirmer test value of <10 mm in 5 minutes, a TFBUT <10 seconds, and the presence of any keratoconjunctival staining (Oxford grading scheme >0) in either eye (Table 1). Control subjects were considered those who had an OSDI ≤7, Schirmer test value of ≥10 mm in 5 minutes, TFBUT of ≥10 seconds, and no keratoconjunctival staining. Corneal fluorescein staining was graded according to the Oxford grading scheme based on the number of fluorescein dots on a 5-point scale in 5 different zones (no dot=0; 1 to 5 dot =1; 6 to 15 dots=2; 16 to 30 dots=3; and >30 dots=4). One additional point was added if there was an area of confluent staining, and two were added if there were ≥2 areas of confluence or if filamentary keratitis was present. Subjects were excluded from the study if they were using topical or systemic medications known to suppress MMP-9, or if they had allergies, a chronic inflammatory or infectious process, or a recent history of ocular injury, trauma, contact lens use, or surgery. DE patients were tested with the InflammaDry test on the one eye with the greatest corneal staining or, if corneal staining was equal in both eyes, the eye with the lowest Schirmer test value. In 143 patients considered to have DE and 63 controls, InflammaDry was found to have a sensitivity of 85% (121 of 143 patients) and specificity of 94% (59 of 63 subjects) using the inclusion criteria as the standard for comparison.39
Table 1.
Dry Eye Disease Severity Grading
| Clinical Testing | Negative Control |
Mild Grade 1 |
Moderate Grade 2 |
Moderately Severe Grade 3 |
Severe Grade 4 |
|---|---|---|---|---|---|
| OSDI score | <13 | ≥13 | ≥13 | ≥13 | ≥13 |
| TFBUT, s | >10 | ≤10 | ≤10 | ≤5 | 0 (Immediate) |
| Staining (0–5) | 0 (None) | 0 (None) | 1 to 2 | 3 | ≥4 |
| Schirmer, mm/5 min | >10 | ≤10 | ≤10 | ≤5 | ≤2 |
Another study by Sambursky et al of 237 patients with DE using a different definition for disease (at least one self-reported symptom of DE on OSDI) were tested for the presence and severity of DE based on fluorescein TFBUT, corneal fluorescein staining, and Schirmer test value. Corneal fluorescein staining was graded according to the aforementioned Oxford grading system. The InflammaDry test was found to have an 81% sensitivity and 98% specificity with the determination of DE defined as the presence of subjective symptoms of dry eye accompanied by at least one of the following objective confirmatory clinical signs: reduced Schirmer test value, reduced TFBUT, or the presence of corneal staining.5
In a recent study by Schargus et al, 20 elderly patients without a prior diagnosis of DE were evaluated with InflammaDry as well as with the OSDI questionnaire, tear osmolarity measurement, TFBUT, corneal fluorescein staining, conjunctival lissamine staining, Schirmer test, and MMP-9 levels as measured from Schirmer strips analyzed by ELISA. DE was defined in two ways: 1) by symptoms alone (OSDI ≥10) and 2) by having at least two of the following: OSDI ≥10, Schirmer ≤10, corneal staining ≥1, conjunctival staining ≥1, and TFBUT ≤7. Of the 9 patients who were determined to have DE by symptoms alone, only 1 tested positive for MMP-9 by InflammaDry. Of the 14 patients who were determined to have DE by symptoms and signs, 2 were positive for MMP-9. The InflammaDry results were confirmed by MMP-9 ELISA testing, and no false positives were detected. Patients testing negative by InflammaDry were confirmed to have an MMP-9 level <40 ng/mL, confirming the relative accuracy of the test.40
In our own recent study, 128 patients with symptoms of DE as determined by the DEQ5 questionnaire (DEQ5 ≥6) were evaluated by InflammaDry in addition to tear osmolarity, TFBUT, corneal staining, Schirmer test, and meibomian gland assessment.41 Of the 128 patients, 39% (n=50) tested positive for MMP-9 in either eye. When DE symptoms and signs were compared by MMP-9 status, no significant differences were noted between the groups. We therefore concluded that clinically significant ocular surface inflammation cannot be predicted by clinical examination alone.41
In summary, these findings suggest that not all patients with symptoms and signs of DE have MMP-9 levels >40 ng/mL. These findings are in contrast to Sambursky’s work,5,39 where over 80% of patients with DE tested positive with InflammaDry. Differences in study population demographics (mean age 53 years in the Sambursky study5 versus 72 years in Schargus study40) and DE definitions may underlie these noted differences.
VII. Future Directions
Many questions remain unanswered with regard to the utility of InflammaDry in evaluation of DE. First, it is unknown whether MMP-9 levels are elevated in all forms of ocular surface disease associated with inflammation. MMP-9 has been shown to be elevated in a number of conditions, including conjunctivochalasis and vernal keratoconjunctivitis, and with contact lens use.26,29,42 The frequencies of MMP-9 elevation in these respective groups of patients remain unknown.
Second, as all reports tested InflammaDry on subjects at a single point in time, the variability of this measure over time is unknown. This is important as other diagnostic techniques for DE (TFBUT, Schirmer scores) demonstrate temporal variation and do not consistently improve with improvements in clinical symptoms.43 The repeatability of InflammaDry results thus requires further investigation.
Third, it is unclear what causes DE symptoms in subjects who are negative for significant MMP-9 levels. Possible explanations may be that inflammation on the ocular surface is not necessary for all DE symptoms, that inflammation could be expressed through a pathway not tested, and/or that InflammaDry may not detect small but potentially clinically relevant increases in MMP-9 that can be detected by conventional high sensitivity immunoassays. Data in support of the first possibility include the finding that many patients with DE report features of neuropathic ocular pain. These include spontaneous pain, dysesthesia (unpleasant, abnormal sensation), hyperalgesia (exaggerated pain response to suprathreshold noxious stimuli), and allodynia (pain response to normally non-noxious stimuli). Neuropathic pain does not require the initial stimulus to be present. Instead, ongoing pathology in any part of the corneal somatosensory pathway from the cornea to the trigeminal nucleus to the cerebral cortex can result in DE symptoms.44 Thus, one hypothesis is that inflammation is critical in the initial phase of disease, but once the corneal somatosensory pathway becomes sensitized, its dysfunction may underlie symptoms even once the inflammation has resolved. On the other hand, alternative mediators from the inflammatory pathway, as described in Section II, may explain DE in InflammaDry-negative patients. For example, lactoferrin, an endogenous anti-inflammatory protein, has been shown to be diminished in DE.45 Conversely, various pro-inflammatory cytokines, such as IL-1 and TNF-α, have been found to be elevated in DE.46 Finally, as mentioned in the previous section, MMP-9 levels <40 ng/mL register as a negative result on the InflammaDry apparatus; thus, subthreshold levels in DE patients are not captured using the InflammaDry test.
Fourth, the inter-relationship between InflammaDry testing and other parameters of DE is not clear. For example, one-third of DE patients with a positive InflammaDry test had no corneal staining.5 More data will be needed on the relationships between MMP-9 levels and other ocular surface measures of DE over time to understand their temporal pattern. For example, it is possible that MMP-9 elevation develops prior to corneal staining, as discussed in Section II of this review. Support for this comes from data finding a stronger correlation between MMP-9 activity and symptoms, as compared with corneal staining.8 As noted above, it is possible that MMP-9 leads to subclinical perturbations in the corneal epithelial barrier, leading to the clinical symptoms of DE prior to noted signs of disease. Another interesting category of patients comprises those with +MMP-9 levels but without DE symptoms. These patients may be at higher risk of developing worsening DE symptoms and signs after surgery (e.g., cataract, refractive error) and may have less reliable presurgical test results (e.g., keratometry, topography, and aberrometry). Data are needed with regard to whether such patients would benefit from an alternation in the presurgical treatment regimen.
Fifth, questions remain regarding the predictive value of MMP-9 on the treatment of DE. If MMP-9 positive patients are indeed found to respond more favorably to anti-inflammatory therapies, this will help tailor therapy in DE sufferers. The ability to discern between DE of an inflammatory versus non-inflammatory etiology may aid in treatment decisions: anti-inflammatory treatments including cyclosporine, corticosteroid, or doxycycline versus more conservative options such as artificial tears and ointments. Furthermore, some procedures, such as punctal occlusion could be avoided in those with ocular surface inflammation, as increased retention time of tears with inflammatory cytokines may aggravate eye inflammation.47 If InflammaDry is prognostic of a response to therapy, it also has the potential to help monitor the resolution of inflammatory DE.
Sixth, the role of InflammaDry in the management of ocular surface problems associated with ocular diseases such as glaucoma needs further study. MMP-9 activity promotes uveoscleral outflow and therefore is the desired goal of glaucoma treatment. Prostaglandin analogs upregulate MMP expression and have been found to affect corneal morphology, with significant corneal thinning compared to baseline (P=.03) as a side effect of treatment.48,49 Surveillance of corneal surface MMP-9 may help prevent permanent corneal damage and sway clinicians to use alternative treatments for glaucoma.
VIII. Conclusion
DE likely comprises a collection of disparate diseases, each with its own unique etiology, and InflammaDry may clarify one more piece of the puzzle to help identify these differences. However, more studies are needed to elucidate all the factors involved, allowing the clinician to understand how to best incorporate InflammaDry into the DE testing regimen to aid in diagnosis and appropriately guide treatment decisions.
Acknowledgments
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development’s Career Development Award CDA-2-024-10S (Dr. Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors note that the contents of this study do not represent the views of the Department of Veterans Affairs or the United States Government.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. (No authors listed) [DOI] [PubMed] [Google Scholar]
- 2.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. (No authors listed) [DOI] [PubMed] [Google Scholar]
- 3.Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21:310–6. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 4.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23:762–70. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 5.Sambursky R, Davitt WF, 3rd, Friedberg M, Tauber S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. 2014;33:812–8. doi: 10.1097/ICO.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 6.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–9. doi: 10.1016/s0161-6420(99)00176-1. Erratum: Ophthalmology 2000;107:1220. [DOI] [PubMed] [Google Scholar]
- 7.McCabe E, Narayanan S. Advancements in anti-inflammatory therapy for dry eye syndrome. Optometry. 2009;80:555–66. doi: 10.1016/j.optm.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–9. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aragona P, Aguennouz M, Rania L, et al. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology. 2015;122:62–71. doi: 10.1016/j.ophtha.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 10.Pflugfelder SC. Anti-inflammatory therapy of dry eye. Ocul Surf. 2003;1:31–6. doi: 10.1016/s1542-0124(12)70005-8. [DOI] [PubMed] [Google Scholar]
- 11.Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82:885–98. doi: 10.1016/j.exer.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng HC, Lee IT, Lin CC, et al. IL-1beta promotes corneal epithelial cell migration by increasing MMP-9 expression through NF-kappaB- and AP-1-dependent pathways. 2013;8:e57955. doi: 10.1371/journal.pone.0057955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9:240–50. [PMC free article] [PubMed] [Google Scholar]
- 14.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CC, Kuo CT, Cheng CY, et al. IL-1 beta promotes A549 cell migration via MAPKs/AP-1- and NF-kappaB-dependent matrix metalloproteinase-9 expression. Cell Signal. 2009;21:1652–62. doi: 10.1016/j.cellsig.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–90. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corrales RM, Stern ME, De Paiva CS, et al. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3293–302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 19.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 20.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31:186–93. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 21.Sambursky R, O'Brien TP. MMP-9 and the perioperative management of LASIK surgery. Curr Opin Ophthalmol. 2011;22:294–303. doi: 10.1097/ICU.0b013e32834787bb. [DOI] [PubMed] [Google Scholar]
- 22.VanDerMeid KR, Su SP, Krenzer KL, et al. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol Vis. 2011;17:1056–63. [PMC free article] [PubMed] [Google Scholar]
- 23.VanDerMeid KR, Su SP, Ward KW, Zhang JZ. Correlation of tear inflammatory cytokines and matrix metalloproteinases with four dry eye diagnostic tests. Invest Ophthalmol Vis Sci. 2012;53:1512–8. doi: 10.1167/iovs.11-7627. [DOI] [PubMed] [Google Scholar]
- 24.Barton K, Monroy DC, Nava A, Pflugfelder SC. Inflammatory cytokines in the tears of patients with ocular rosacea. Ophthalmology. 1997;104:1868–74. doi: 10.1016/s0161-6420(97)30014-1. [DOI] [PubMed] [Google Scholar]
- 25.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. [PubMed] [Google Scholar]
- 26.Acera A, Vecino E, Duran JA. Tear MMP-9 levels as a marker of ocular surface inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2013;54:8285–91. doi: 10.1167/iovs.13-12235. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi A, Brun P, Abatangelo G, et al. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44:3052–8. doi: 10.1167/iovs.02-0766. [DOI] [PubMed] [Google Scholar]
- 28.Lema I, Sobrino T, Duran JA, et al. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol. 2009;93:820–4. doi: 10.1136/bjo.2008.144253. [DOI] [PubMed] [Google Scholar]
- 29.Markoulli M, Papas E, Cole N, Holden B. Effect of contact lens wear on the diurnal profile of matrix metalloproteinase 9 in tears. Optom Vis Sci. 2013;90:419–29. doi: 10.1097/OPX.0b013e31828d7d3b. [DOI] [PubMed] [Google Scholar]
- 30.Sobrin L, Liu Z, Monroy DC, et al. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000;41:1703–9. [PubMed] [Google Scholar]
- 31.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–35. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Mori M, De Lorenzo E, Torre E, et al. A highly soluble matrix metalloproteinase-9 inhibitor for potential treatment of dry eye syndrome. Basic Clin Pharmacol Toxicol. 2012;111:289–95. doi: 10.1111/j.1742-7843.2012.00896.x. [DOI] [PubMed] [Google Scholar]
- 33.Dursun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13. doi: 10.1016/s0002-9394(01)00913-8. [DOI] [PubMed] [Google Scholar]
- 34.Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–57. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 35.Foulks GN. Topical cyclosporine for treatment of ocular surface disease. Int Ophthalmol Clin. 2006;46:105–22. doi: 10.1097/01.iio.0000212135.77675.6a. [DOI] [PubMed] [Google Scholar]
- 36.Macri A, Rolando M, Pflugfelder S. A standardized visual scale for evaluation of tear fluorescein clearance. Ophthalmology. 2000;107:1338–43. doi: 10.1016/s0161-6420(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 37.Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118:1489–96. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 38.Gurdal C, Genc I, Sarac O, et al. Topical cyclosporine in thyroid orbitopathy-related dry eye: clinical findings, conjunctival epithelial apoptosis, and MMP-9 expression. Curr Eye Res. 2010;35:771–7. doi: 10.3109/02713683.2010.490320. [DOI] [PubMed] [Google Scholar]
- 39.Sambursky R, Davitt WF, 3rd, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013;131:24–8. doi: 10.1001/jamaophthalmol.2013.561. [DOI] [PubMed] [Google Scholar]
- 40.Schargus M, Ivanova S, Kakkassery V, et al. Correlation of tear film osmolarity and 2 different MMP-9 tests with common dry eye tests in a cohort of non-dryeye patients. Cornea. 2015;34:739–44. doi: 10.1097/ICO.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 41.Lanza NL, McClellan A, Batawi H, et al. Dry eye profiles in patients with a positive elevated surface matrix metalloproteinase 9 point-of-care test versus negative patients. Ocul Surf. 2016;14 doi: 10.1016/j.jtos.2015.12.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumagai N, Yamamoto K, Fukuda K, et al. Active matrix metalloproteinases in the tear fluid of individuals with vernal keratoconjunctivitis. J Allergy Clin Immunol. 2002;110:489–91. doi: 10.1067/mai.2002.126379. [DOI] [PubMed] [Google Scholar]
- 43.Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–85. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10:2–14. doi: 10.1016/j.jtos.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 45.McCollum CJ, Foulks GN, Bodner B, et al. Rapid assay of lactoferrin in keratoconjunctivitis sicca. Cornea. 1994;13:505–8. [PubMed] [Google Scholar]
- 46.Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–11. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 47.Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–7. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 48.Honda N, Miyai T, Nejima R, et al. Effect of latanoprost on the expression of matrix metalloproteinases and tissue inhibitor of metalloproteinase 1 on the ocular surface. Arch Ophthalmol. 2010;128:466–71. doi: 10.1001/archophthalmol.2010.40. [DOI] [PubMed] [Google Scholar]
- 49.Sen E, Nalcacioglu P, Yazici A, et al. Comparison of the effects of latanoprost and bimatoprost on central corneal thickness. J Glaucoma. 2008;17:398–402. doi: 10.1097/IJG.0b013e31815d784c. [DOI] [PubMed] [Google Scholar]