Figure 7. Model for a critical role of SENP1-mediated deSUMOylation in the regulation of endothelial NOTCH1 and angiogenesis.
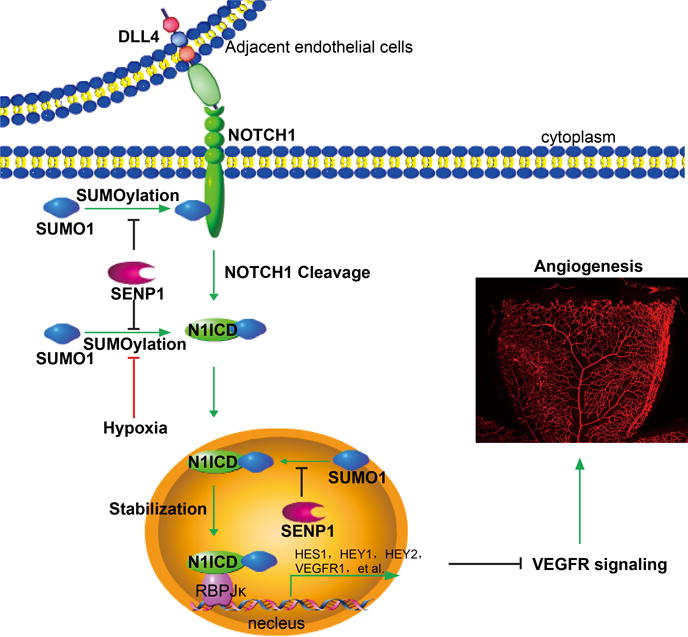
The endothelial cell Dll4-activated NOTCH signal pathway requires SUMOylation of N1ICD for NOTCH1 cleavage to occur, which releases N1ICD from the cell membrane, stabilizes N1ICD and initiates substantial co-transcriptional activity in the nucleus. SENP1 associates with N1ICD of NOTCH1 and deconjugates the SUMO modification as an intrinsic controller, whereas NOTCH1 SUMOylation facilitates dynamic NOTCH responses and subsequent angiogenic homeostasis regulated by hypoxia. In the case of endothelial SENP1 deficiency or functional mutation, augmented N1ICD SUMOylation leads to exaggerated NOTCH signaling, which in turn suppresses VEGFR signaling and endothelial angiogenesis.
