Figure 6.
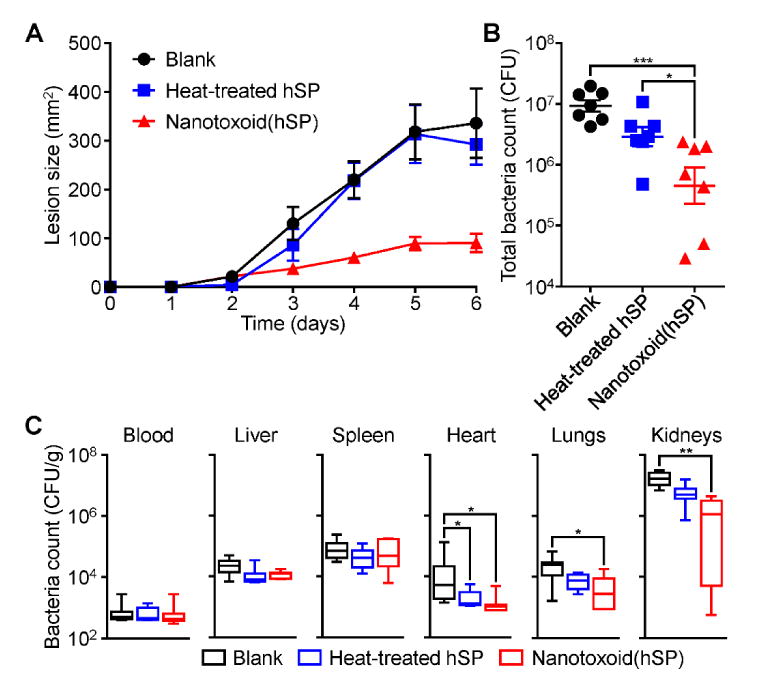
Protection against challenge with live bacteria. Mice were vaccinated with blank solution, heat-treated hSP, or nanotoxoid(hSP) on day 0 with boosts on days 7 and 14. A) Lesion size over time after subcutaneous challenge with MRSA USA300 on day 35 (n = 7; mean ± SEM). B) Total bacterial load summed from major organs 3 days after intravenous challenge with MRSA USA300 on day 35 (n = 7; geometric mean ± SEM). C) Individual, weight-normalized bacteria counts in major organs from (B) (n = 7; min to max). * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA.
