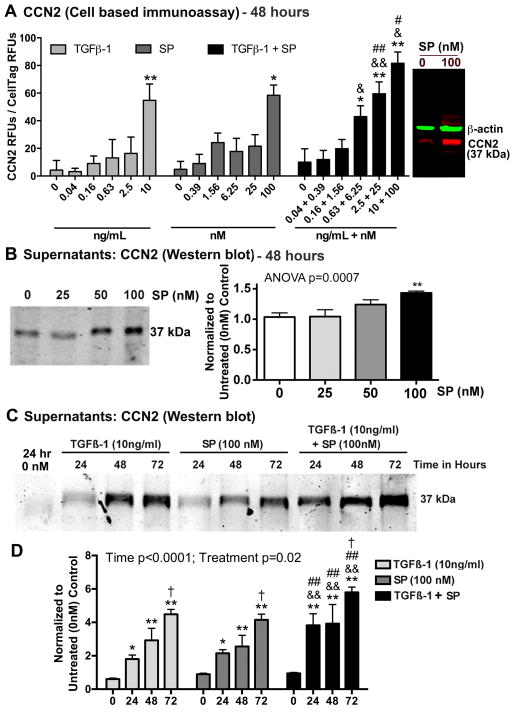
Figure 4.
Both SP and TGFβ-1 increase expression of CCN2 in cultured rat tenocytes in dose dependent manners. Equal numbers of passage 3–5 tenocytes were plated and grown to 90% confluence, serum restricted (1–2% FBS) for 24 hours, before treatment with TGFβ-1, SP, or their combination, at indicated concentrations for 24, 48 or 72 hours. (A) CCN2 production was assessed after 48 hours using quantitative cell-based immunoassay methodology. Data are the means of six independent experiments ± SEM. Gel figure on right is a representative Western blot showing specificity of antibody used for this assay. Molecular weight of CCN2 detected was 37 kDa and β-actin was 42 kDa. (B) Representative Western blot of tenocyte supernatants probed with anti-CCN2 after stimulating with SP at indicated concentrations for 48 hours. Right: Densitometric results from three independent experiments ± SEM. CCN2 band was normalized to 48 hour vehicle control levels (PBS and 1% BSA; “0”). (C) Representative Western blot of tenocyte supernatants probed with anti-CCN2, after stimulation with TGFβ-1, SP, or their combination for indicated hours. (D) Densitometric results of Western blots from four independent experiments ± SEM. CCN2 band was normalized to time-matched vehicle control levels (PBS and 1% BSA). ANOVA results shown panels A, C, and D. * and **: p<0.05 and p<0.01, respectively, compared to vehicle controls; & and &&: p<0.05 and p<0.01, compared to TGFβ-1 treatment; # and ##: p<0.05 and p<0.01, compared to SP treatment; †: p<0.05, compared to 24-hour time-point.