Fig. 1.
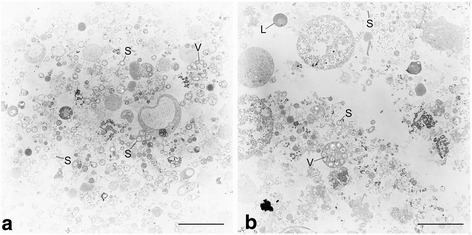
Transmission electron micrographs of Candidatus Thioglobus thermophilus. B. thermophilus gill tissue was homogenized in a glass tissue grinder and subjected to crude density gradient centrifugation using Histodenz™ gradient medium. Subsamples were taken from two visible bands and fixed for electron microscopy (a and b). Both subsamples contained numerous free symbiont cells (S) as well as some intact host vacuoles (V) containing several symbiont cells, besides various other cellular components and host tissue debris. L: Lipid drop or mucus. Scale bar: 5 μm. Electron microscopy method details: samples were fixed in a) 1% glutaraldehyde, 2% paraformaldehyde in IBS (imidazole-buffered saline; 0.49 M NaCl, 30 mM MgSO4*7H2O, 11 mM CaCl2*2H2O, 3 mM KCl, 50 mM imidazole) and b) in 2.5% glutaraldehyde, 1.25% paraformaldehyde in IBS. After embedding in low-gelling agarose and postfixation in 1% osmium tetroxide in cacodylate buffer (0.1 M cacodylate; pH 7.0), samples were dehydrated in a graded ethanol series (30 to 100%) and embedded in a mixture of Epon and Spurr (1:2). Sections were cut on an ultramicrotome (Reichert Ultracut, Leica UK Ltd., Milton Keynes, UK), stained with 4% aqueous uranyl acetate for 5 min followed by lead citrate for 1 min and analyzed with a transmission electron microscope LEO 906 (Zeiss, Oberkochen, Germany)
