Figure 2. Prmt1 is required for tumor initiation driven by p53/Rb loss.
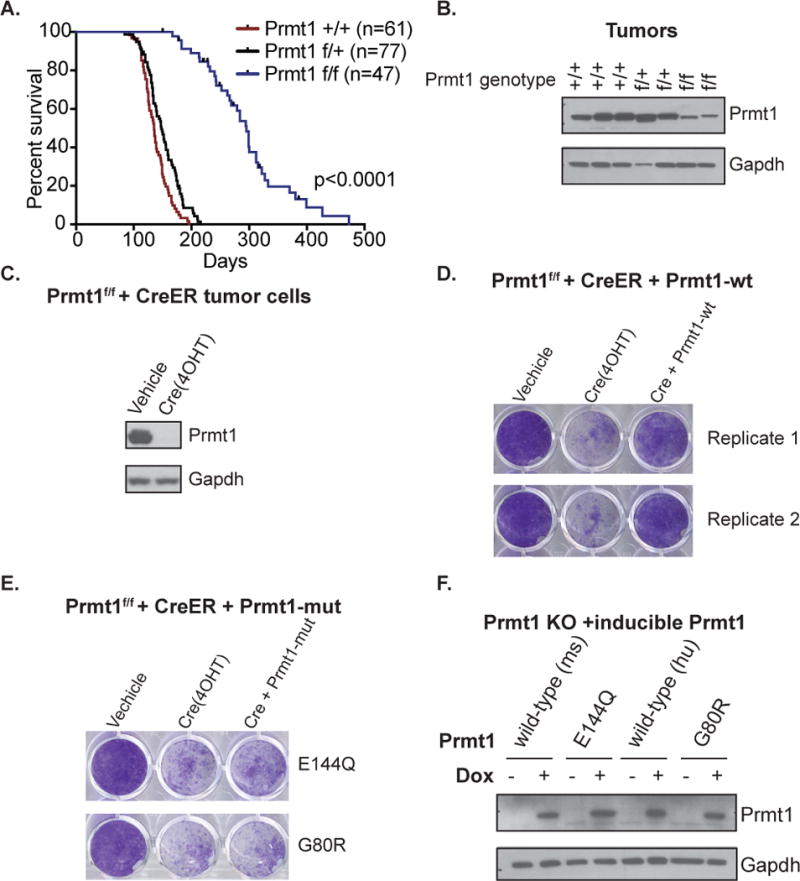
(A) Conditional knockout Prmt1f/f mice were crossed to OsxCre+, p53f/f; Rbf/f to generate OsxCre+, p53f/f; Rbf/f; Prmt1f/f mice. Kaplan-Meier survival plot and summary of median survival of Prmt1+/+, Prmt1+/f, and Prmt1f/f OS mice. Logrank test was performed for comparison. *p<0.0001 (B) Western blot analysis of Prmt1 protein expression in Prmt1+/+, Prmt1 +/f, and Prmt1f/f p53/Rb-null OS tumors. Gapdh expression serves as the loading control. (C) Western blot analysis of Prmt1 protein expression in Cre-ER expressing tumor derived Prmt1f/f p53/Rb-null mOS cell lines following vehicle (Ethanol) or tamoxifen (4OHT) treatment. (D) Proliferation assessment of conditional Prmt1 KO (Prmt1f/f CreER) cells expressing a dox-inducible wild-type Prmt1 following vehicle, 4OHT, or both 4OHT and dox treatment by crytal violet staining. (E) Proliferation assessment of conditional Prmt1 KO (Prmt1f/f CreER) cells expressing a dox-inducible catalytic point mutant Prmt1 (E144Q or G80R) following vehicle, 4OHT, or both 4OHT and dox treatment by crytal violet staining. Representative images of the three replicate experiments are shown.
(F) Western blot analysis of doxycycline-inducible murine wild-type Prmt1 (mPrmt1), Prmt1 catalytic mutant (E144Q), human wild-type Prmt1 (hPrmt1), and Prmt1 catalytic mutant (G80R) in 4OHT treated Prmt1 conditional knockout p53/Rb-null mOS cells (Prmt1f/f CreER).
