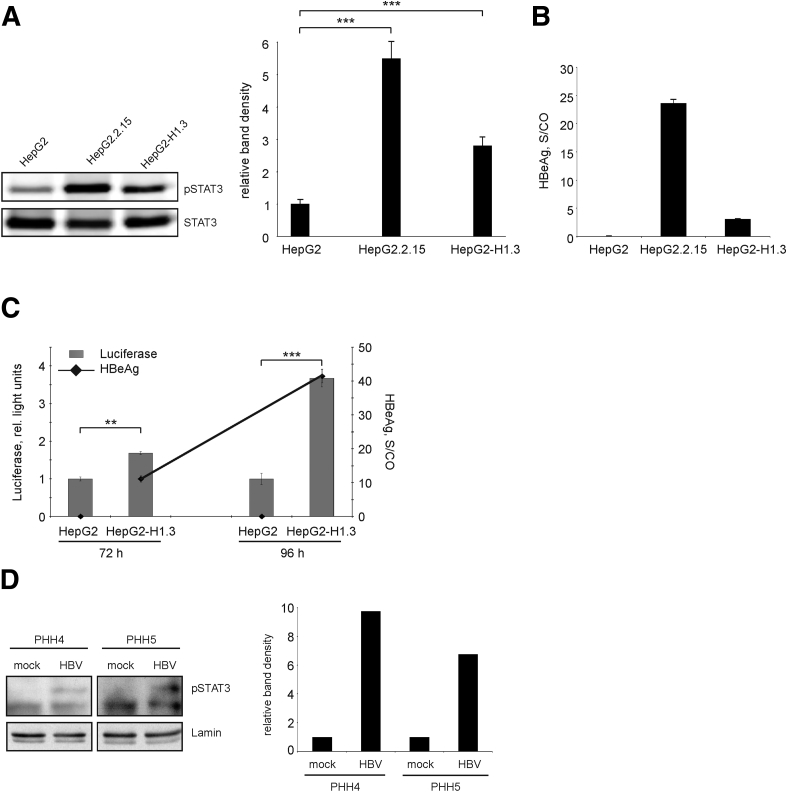
Figure 3.
Activation of signal transducer and activator of transcription 3 (STAT3) in hepatitis B virus (HBV) replicating cells. (A) Total cellular proteins were analyzed for the presence of tyrosine 705 phosphorylated STAT3 (pSTAT3) and total STAT3 by Western blotting. One representative Western blot of 3 is shown (left panel). Band density was quantified using ImageJ software and relative levels of pSTAT3 were calculated (right panel). Level of pSTAT3 in HepG2 cells was set to 1. Values are shown as mean ± SD (n = 3; ***P < .001; Student's t test). (B) Secretion of hepatitis B early antigen (HBeAg) into the medium of stably HBV-replicating HepG2.215 or HepG2-H1.3 cells was determined as signal-to-control (S/CO) ratio, mean ± SD from 3 independent experiments is given. HepG2 cells were used as negative control. (C) Activation of STAT3 in HepG2-H1.3 cells was analyzed by the STAT3-luciferase reporter assay. HepG2-H1.3 and HepG2 cells were transfected with STAT3 Cignal reporter, negative or positive control constructs. Cells were harvested 72 and 96 hours after transfection and analyzed by the Dual-Luciferase Cignal reporter assay. The activity of STAT3-dependent firefly luciferase is expressed in relative light units. The activity of constitutively expressed Renilla luciferase was used for internal normalization. Secretion of HBeAg into the culture medium of HepG2-H1.3 cells is indicated by dots. Values are shown as mean ± SD (n = 3; **P < .01; ***P < .001; Student's t test). (D) Nuclear proteins extracted from mock- or HBV-infected PHHs prepared from 2 different donors (PHH4 and PHH5) on day 4 postinfection were analyzed for the presence of pSTAT3 by Western blotting (left panel). Band densities for pSTAT3 and lamin (loading control for nuclear proteins) were quantified using ImageJ software (right panel), and levels of pSTAT3 were calculated relative to respective mock control (set as 1).