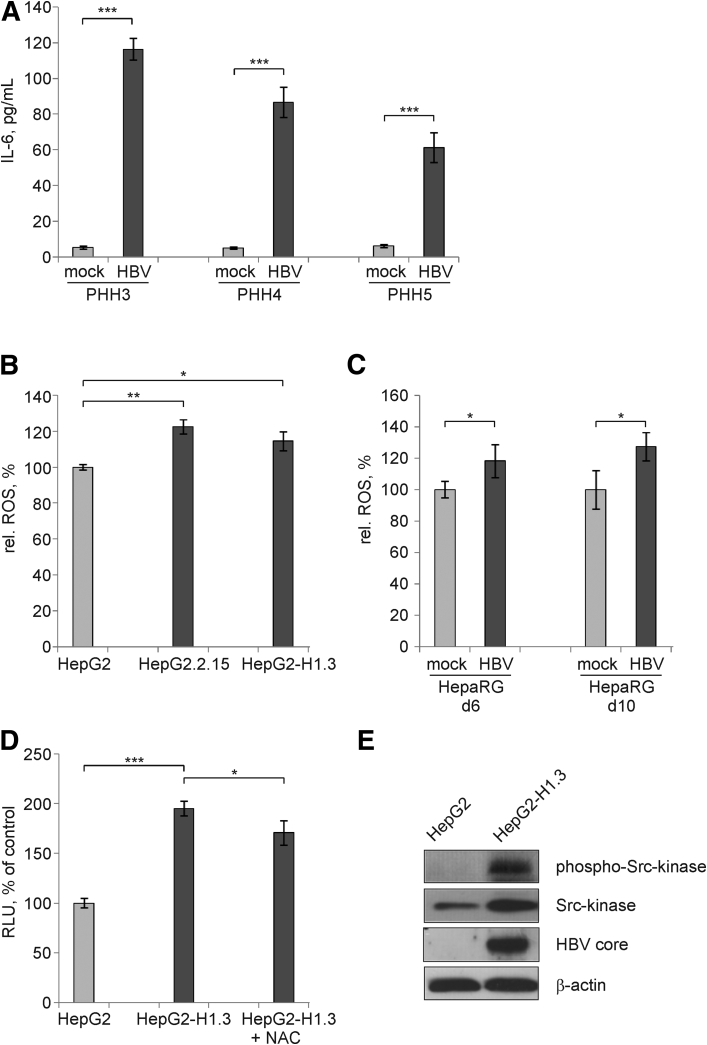
Figure 6.
Activation of signal transducer and activator of transcription 3 (STAT3) by interleukin 6 (IL-6)–dependent and –independent intracellular mechanisms. (A) Secretion of IL-6 into the medium of mock- or HBV-infected primary human hepatocyte (PHH) cultures prepared from 3 different patients was measured by enzyme-linked immunosorbent assay (ELISA) on day 4 p.i. Values are shown as mean ± SD (n = 3; ***P < .001; Student's t test). (B) Reactive oxygen species (ROS) were determined in HepG2, HepG2.2.15, and HepG2-H1.3 cells on day 7 after reaching confluency and (C) in mock- or HBV-infected HepaRG cells on day 6 and 10 p.i. by Cellular ROS Detection Assay Kit. Relative ROS levels in HepG2 cells or in mock-infected HepaRG cells were set to 100%. Values are shown as mean ± SD (n = 6; *P < .05; **P < .01; Student's t test). (D) Levels of STAT3 activity after treatment of HepG2-H1.3 cells with 5 mM of ROS-inhibitor N-acetyl-L-cysteine (NAC) were determined by the STAT3-luciferase reporter assay. Relative activity of firefly luciferase reflecting STAT3 activation in HepG2 cells was set to 100%. Values are shown as mean ± SD (n = 6; *P < .05; ***P < .001; Student's t test). (E) Phosphorylated (Tyr416) and nonphosphorylated Src-kinase were detected by Western blot analysis using total cellular proteins isolated from HepG2 or HepG2-H1.3 cells. To control HBV replication and protein loading, membranes were reprobed and stained with anti-HBV core or β actin antibodies, respectively. RLU, relative light units.