Figure 3.
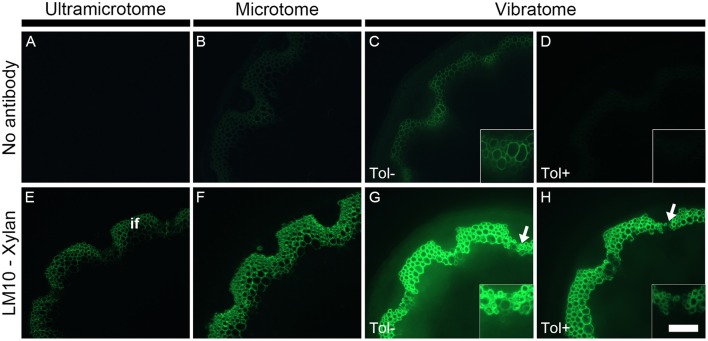
The detection of primary fluorescence and indirect immunolabeling relies on the preparation of plant samples. Sections cut with either an ultramicrotome (A,E), a microtome (B,F) or a vibratome (C,D,G,H) were obtained from equivalent stem regions (ca. 15 cm distal from the rosette of stems with an average height of 30 cm) and photographed by applying the exact same parameters (e.g., same time of exposure). Micrographs (A–D) show the primary fluorescence of equivalent transverse stem sections of A. thaliana under blue excitation. This fluorescence is naturally emitted, for example, by phenolic compounds such as the lignin present in the interfascicular fibers (if). Micrographs (E–H) show the indirect immunolabeling of xylan, a cell wall polysaccharide recognized by the LM10 monoclonal antibody. The primary antibody binding is revealed with a secondary anti-rat monoclonal antibody coupled with a FITC fluorochrome. The intensity of both primary fluorescence and the artificial fluorescence resulting from the immunolabeling are proportional to the thickness of the sections. As shown here, both type of fluorescence increase with section thickness. The sections obtained with a vibratome were used to demonstrate the effect of toluidine blue when the sections are excited under blue light. When overlapping with the artificial fluorescence, the primary fluorescence in absence of toluidine blue (Tol −) (C,G and their insets) can impede the interpretation of immunolabeling. A staining with toluidine blue (Tol +) (D,H) drastically reduces the autofluorescence (C vs. D) with, in this example, a minimal loss of epitope detection (G vs. H, insets). The arrows in micrographs (G,H) highlight regions where the LM10 antibody bound weakly to cell walls when the sections were stained after immunolabeling. Scale bar: for the large micrographs: 100 μm, for the insets: 50 μm.