Figure 4.
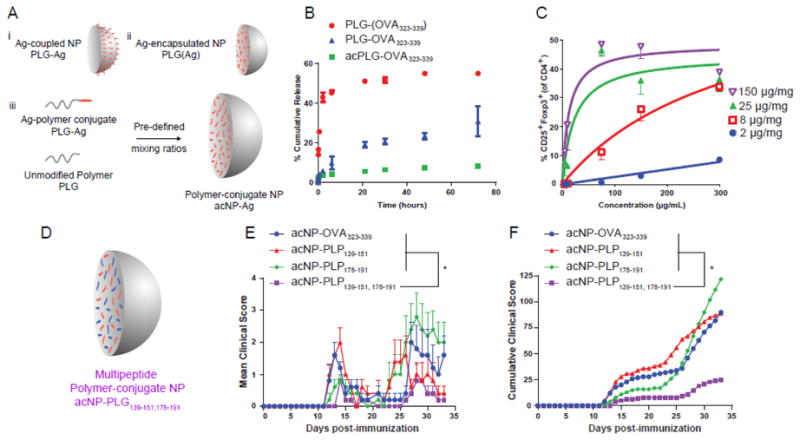
Antigen-polymer conjugate nanoparticles display favorable physicochemical and biological properties for tolerance induction. (A) Schematic representation of Ag-coupled, Ag-encapsulated, and polymer-conjugate nanoparticles. (B) Release profile of NP(OVA323-339), NP-OVA323-339, and acNP-OVA323-339. (C) Regulatory T cell induction is dependent on nanoparticle concentration. BMDCs were treated for 3 hr with various concentrations of acNP-OVA323-339 (2, 8, 25, 150 μg/mg loading). Excess acNP-OVA323-339 particles were subsequently washed from the cell surface prior to addition of OT-II T cells and 2 ng/mL of TGF-β1. (D) Schematic representation of antigen-polymer conjugate nanoparticles delivering multiple Ags. (E) Clinical scores of SJL/J mice treated with 1.25 mg of acNP-OVA323-339 (8 μg/mg OVA323-339), acNP-PLP139-151 (8 μg/mg PLP139-151), acNP-PLP178-191 (8 μg/mg PLP178-191), or acNP-PLP139-151,178-191 (8 μg/mg PLP139-151 and 8 μg/mg PLP178-191) and immunized with PLP139-151 and PLP178-191 in CFA to induce R-EAE 7 days later. (F) Corresponding cumulative clinical score for mice treated with particles (n = 5). Differences between disease courses of different treatment groups were analyzed for statistical significance using the Kruskal-Wallis test (one-way ANOVA non-parametric test) with Dunn's multiple comparisons test (p < 0.05) [94].
