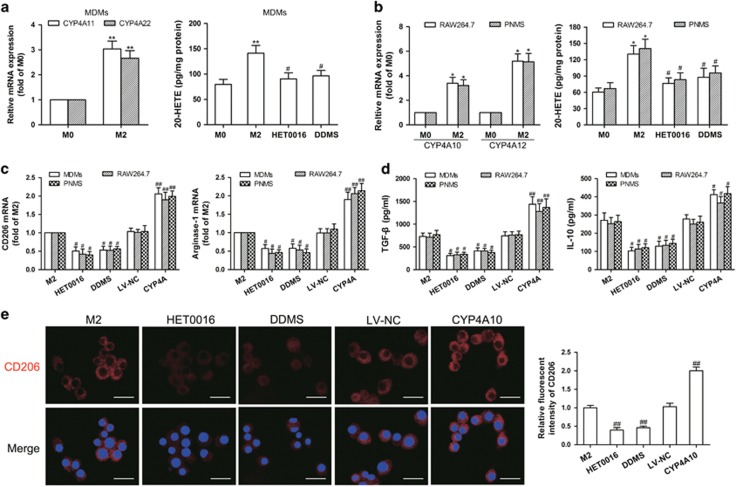
Figure 6.
CYP4A contributes to macrophage M2 polarization in vitro. (a) Human monocyte-derived macrophages (MDMs) were treated with the tumor-conditioned medium (CM) for 72 h to generate M2 macrophages (M2), and then the unpolarized macrophages (M0) and M2 were incubated with HET0016 (5 μM), DDMS (10 μM) or vehicle for 24 h. CYP4A11/22 mRNA expression in the M0 and M2 was measured by qPCR, and 20-HETE production in M0, M2 and HET0016 or DDMS-treated M2 was determined by LC-ESI-MS/MS. (b) RAW264.7 cells and mouse peritoneal macrophages (PNMS) were treated with IL-4/IL-13 (20 ng/ml) for 12 h to generate M2, and then the M0 and M2 were incubated with HET0016 (5 μM), DDMS (10 μM) or vehicle for 24 h. CYP4A10/12 mRNA expression in the M0 and M2 was measured by qPCR, and 20-HETE production in M0, M2 and HET0016 or DDMS-treated M2 was determined by LC-ESI-MS/MS. (c) Parental and CYP4A10/11high macrophages treated with IL-4/IL-13 (20 ng/ml) for 12 h or the tumor CM for 72 h were incubated with HET0016 (5 μM), DDMS (10 μM) or vehicle for 24 h. M2 markers (CD206 and arginase-1) were determined by qPCR. (d) Production of Th2 cytokines (TGF-β and IL-10) was determined by ELISA. (e) Immunostaining analysis of CD206 (red) in PNMS from the above treated groups was shown. Scale bars, 20 μm. Each value presents the mean±s.e.m. of three independent triplicate experiments. *P<0.05, **P<0.01 vs M0, #P<0.05, ##P<0.01 vs M2.